When passengers take their luggage through an airport security check, computed tomography technology produces a 3-D image of the contents, allowing security officers to see what’s inside without having to open each bag.
Researchers at Penn State University, sponsored by the Electric Power Research Institute (EPRI), are applying that same sort of non-destructive technology to study corrosion in the power plant industry, only in miniature. They are examining how contaminants in power plant water cycles affect the integrity of steel pipes and tubing in power generation systems.
EPRI sought out the research to validate its boiler and turbine steam cycle chemistry guidelines. The agency chose Penn State (State College, Pennsylvania, USA) because the university has the capacity to simulate boiler water conditions in the lab with temperature, pressure, and water chemistry in addition to state-of-the-art materials characterization instrumentation. For this project they use X-ray computed tomography to quantify corrosion rates by non-destructive means using Instrumentation at Penn State’s Institutes of Energy and the Environment’s Center for Quantitative Imaging.
Derek Hall, an assistant professor of the Department of Mechanical Engineering at Penn State, leads the experimental effort with the help of this doctoral student, Akash Ganesan.
Boiler System Research
A power plant boiler system works by heating water to generate steam, which drives a turbine connected to an electricity generator. The process begins with fuel (such as coal, natural gas, or nuclear material) being burned in the boiler's furnace to produce heat. This heat converts water into high-pressure steam, which flows to the turbine.
As the steam expands through the turbine, it spins the turbine blades, turning the generator and producing electricity. After passing through the turbine, the steam is condensed back into water in a condenser and returned to the boiler to be heated again, completing the cycle.
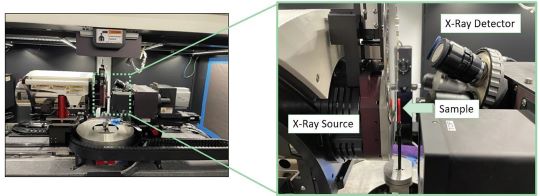
“One of the big benefits of this project is that you can do it in those smaller lab conditions and non-destructively instead of having to cut the test specimen for cross-sectional corrosion analysis,” says EPRI program manager Brad Burns of Charlotte, North Carolina, USA. “We’ve tried to simulate upset boiler chemistry conditions by injecting contamination, namely chloride and sulfate—hydrochloric acid and sulfuric acid—to try to simulate what’s happening right at that metal surface in a boiler tube.”
Hall says scientists know that these contaminants increase the corrosion rate, so their research aims to understand why and how they do so.
Hall, who holds a Ph.D. in energy and mineral engineering from Penn State, explains that to re-create the high-temperature, high-pressure conditions that occur in power plant steam cycles, university researchers use an autoclave that can sustain temperatures as high as 300 °C (572 °F).
“Our technique allows you to quantify the localized corrosion rate, which is typically what is going to cause an issue,” he says. “I think this new monitoring method will provide valuable insights to how the corrosion process occurs under these oxide layers, which has been very difficult to study previously.”
The research can eventually help power plant operators make better decisions about different boiler water treatment strategies and cleaning methodologies for carbon steel. The researchers in this project also believe that the use of CT-scanning may also be beneficial to other industries that rely on corrosion coupons or corrosion coupon studies.
“If you’re using corrosion coupons to monitor corrosion rate, this technique could be a good alternative, because it can provide you more information in shorter timeframes,” Hall says.
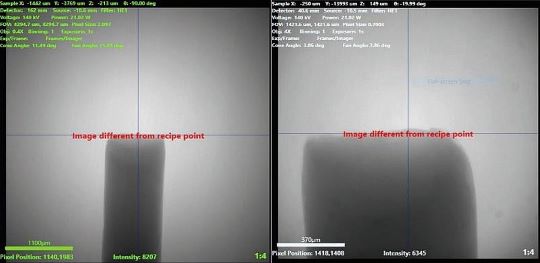
Nondestructive Testing
The research is being conducted with the use of a sophisticated piece of equipment called the Zeiss Versa 620. The nondestructive instrument provides three-dimensional imaging and analysis of a wide range of materials with high contrast and resolution, according to its manufacturer.
It is suitable for a broad spectrum of materials, including metals, polymers, ceramics, and biological specimens, and accommodates a variety of sample sizes and shapes, from small specimens to larger industrial components.
“It gives us a highly detailed 3D image of the oxide layer and base metal corrosion without damaging the sample,” Hall says.
Penn State researchers explain that the Zeiss Versa allows them to achieve a resolution of about 300 nanometers, or 1% the width of a human hair.
“Derek’s team can actually take slices virtually, slice it 1,000 times, and look at damage cross section by cross section,” says Burns, a graduate of the Georgia Institute of Technology, better known as Georgia Tech.
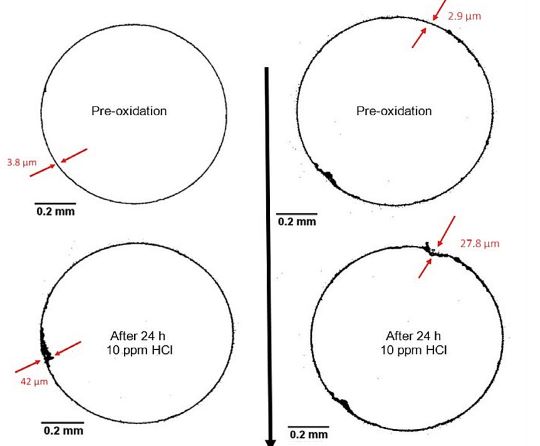
The non-destructive aspect of the work is crucial as well.
“We can use the same corrosion test specimen over and over again,” Burns explains. “Derek’s X-ray method doesn’t destroy it. In the past, you would have to actually cut it and then look at it with a microscope or an SEM to try to determine what’s going on, and then you’ve destroyed the specimen.”
After working on the project for three and a half years, Hall and Burns are moving into a new phase testing a specific corrosion inhibitor.
“It will be interesting to look at these filming type corrosion inhibitors to see how this apparatus might be able to give us insight into how they behave and what they do to inhibit corrosion in that environment,” Burns says.
Renewable Energy Impact
Hall and Burns explain that their work will be especially helpful for thermal power generation systems affected by demand changes resulting from increased renewable energy such as solar and wind. These renewable energy systems are non-dispatchable in nature, requiring thermal plant operators to adjust electrical power outputs to stabilize the grid.
“With more non-dispatchable resources on the grid, like solar and wind, there is much less control of when those assets are available to generate power,” Burns explains. “So, guess who takes the brunt of that? The old thermal plants. They have to cycle way down, sometimes all the way off, when the sun’s bright, and the wind’s blowing. And then when the sun’s not shining, and the wind’s not blowing, thermal plants have to ramp up and meet the demand. That is tougher on corrosion in some areas of the plant.”
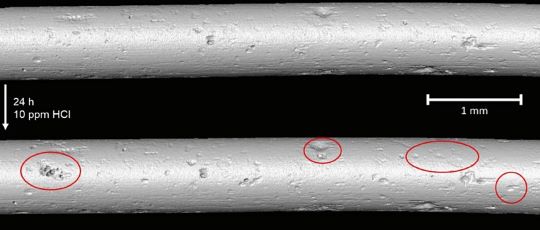
Impact of the Research
The researchers say they want solid scientific evidence in place to help plant managers whose chemistry conditions could result in corrosion damage.
“We’re trying to improve upon what we have already, which I think are unparalleled comprehensive cycle chemistry guidelines for the industry,” EPRI’s Burns says. “We can look at the amount of corrosion and the pits that form under various conditions over various periods of time, to try to enhance what we know about corrosion already and improve upon that state of knowledge and incorporate that into our comprehensive guidelines. This will help power plants lower the risk of these types of failure mechanisms in their steam generator and boiler tubes.”
Penn State’s Hall said a primary objective of the research was to capture corrosion rates that would represent what would happen if a plant operated above a certain level of contamination. Providing that indicator of concern level has not been available previously.
“The first step in addressing any of these corrosion issues is whether we can understand and monitor the process, and after that can we test out methods to solve it or to stop it. The first step is really to be able to understand and observe it clearly and to be able to look under deposits without destroying them. That’s going to provide some truly valuable insights.”
Editor’s note: This article first appeared in the September 2024 print issue of Materials Performance (MP) Magazine. Reprinted with permission.