Naval Research Laboratory Key West is a state-of-the-art testing facility for atmospheric and marine corrosion. A two-phase, multi-year severity assessment was conducted to compare 16 sites to Key West. Data presented will include C1010 steel mass loss and galvanostatic reduction of silver. An understanding of how different local environments impact site-specific corrosion was developed. There is an opportunity to simulate the environments of other bases at Key West by altering the Key West environment through various means.
The exposure environment of an engineering material has a large impact on how that material behaves over time. Environments are distinguished by differences in meteorological patterns, geography, salinity, ultraviolet (UV) radiation, etc.1-3
Thus, the degradation of various materials scales proportionately to the characteristics of the exposure site, with more severe sites leading to worse degradation. Developing an understanding of how the local environment impacts the corrosion rates of metals and the deterioration of anticorrosion coatings is critical for informing asset maintenance schedules and lifetime predictions.4
The Naval Research Laboratory (NRL) Key West facility is a rigorously characterized atmospheric corrosion test site. It offers a platform for elucidating the effects of the environment on the atmospheric corrosion of engineering materials.
The NRL Center for Corrosion and Atmospheric Structural Testing (C-CoAST) has the potential to be “tuned” to replicate a variety of service conditions. This is accomplished by the combination of sea water spray, clear water rinsing, static or dynamic loading, covering/sheltering, etc.5
However, to be able to fine-tune the Key West environment, the relationship between environmental parameters, corrosion rates, and coating degradation must be known. To understand how results obtained from Key West will compare to Navy and Marine Corps bases, an update to the historical environmental severity index (ESI) is needed.
This understanding is developed through comparison of the Key West test site with other exposure test sites via witness coupon corrosion and aerospace coating deterioration measurements. A total of 17 sites were assessed, as listed in Table 1.
Sites were selected from aviation-relevant Department of Defense installations across the United States and Japan, as shown in Figure 1. This provided a large parameter space for testing a range of variables. If the NRL Key West site can be successfully modified to mimic the conditions of other sites, then it will confirm that a proper understanding of the effect of environmental conditions on atmospheric corrosion has been achieved.
Furthermore, with this understanding, the NRL Key West site can be modified to mimic other service conditions of interest, greatly increasing the applicability and versatility of Key West. Even if the site cannot be completely adjusted to perfectly mimic other sites, a similar effect can be achieved by adjusting the resulting data using a scaling factor.
Finally, the wide variety of sites assessed in this phase of the program offers a chance to improve our current understanding of how the local environment influences atmospheric corrosion. The climatic/meteorological character of all the sites is diverse, as shown in Figure 1. Locations, Köppen codes, and exposure start dates for each site are listed in Table 1.3
Experimental Procedure
Exposure
The NRL Key West testing facility is located on Fleming Key within the Naval Air Station. It is equipped for structural testing, coating application, and outdoor exposure (both marine submersed and marine atmospheric). Weather and water data are continuously collected to supplement a rich database of environmental parameters measured at the site with the goal of correlating the environment to material performance.
The Key West facility serves as the ground-base or headquarters against which to compare all other sites for this investigation. The other 16 sites consisted of Navy/Marine installations around the United States and Japan (continental U.S. sites shown in Figure 1). The complete list is given in Table 1.
Full details of the study are elaborated in a paper presented at the AMPP Annual Conference + Expo 2022.6 Exposures began over the course of 65 days around December 2020. Table 1 shows the exposure schedule for all 17 sites. In each instance, samples were exposed at the best possible available location and as close to the same time as logistically possible.

Samples
Witness coupons consisted of C1010 low carbon steel (CS) and pure (99.99%) silver. The C1010 is a common proxy sample for steels in general (although it is starkly different from other classes of steel like stainless, cast iron, or high strength steels).
Silver is also a commonly used proxy for monitoring the corrosivity and chemistry of an exposure site.
7-8 The conference proceeding
6 reports details on the experimental procedure and the results of testing on two aerospace coatings of relevance to the naval aviation enterprise. A traditional chrome (VI) coating and a contemporary non-chrome (VI) coating applied over AA2024-T3 were assessed.
Testing
Mass loss measurements were collected from the C1010 low CS coupons, consistent with previous testing at NRL Key West. Mass loss rate in grams per cm2 per day is reported.
Galvanostatic reduction was performed on the silver witness coupons to determine the relative oxidation power of the exposure condition and what atmospheric species were present during exposure.7-8 Results are plotted in terms of total charge and in terms of speciated charge.
Results and Discussion
Witness Coupon Results
C1010 Steel Mass Loss
C1010 is a low CS that corrodes according to a general or uniform mode. The mass loss data is indicative of the corrosion rate of the steel in the various environments as a function of time. Results are reported in full in the conference proceeding.6
Steel mass loss rate results are reported in Figure 2 in the form of a horizontal bar plot (left) and a color-indexed scatter plot (right) overlaid on an outline of the United States. The color corresponds to the one-month mass loss rate from each sample.
As a preliminary analysis, typically the higher mass loss rates were observed for coastal sites. This is not the sole deterministic factor, however, as some coastal sites had moderately low mass, such as Oceana and Iwakuni (Figure 2).
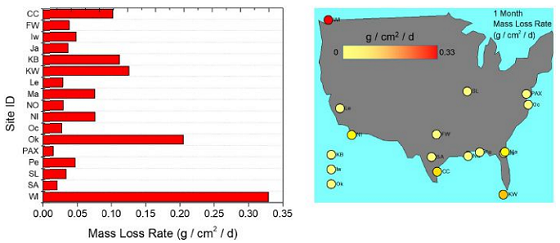
More importantly, the mass loss is likely a function of salty sea spray aerosol production by coastal wave breaking. Chloride deposition from salt aerosols is strongly correlated to C1010 mass loss and varies from location to location, as demonstrated by the Wet Candle Technique or inferred from silver chloride analysis.9
Silver Reduction
Low levels of silver products retained on the surface of the sample can be indicative of either low amounts of oxidation of silver-to-silver cation, washing of the surface so that atmospheric species do not have the chance to react with oxidized silver cations, or potentially photo-reduction of the oxidized species.
Given the almost insoluble nature of common silver compounds and the rather high Nernst potential for silver oxidation, it is more likely that low reduction charge is a result of correspondingly lower levels of silver oxidation. Typically, ultraviolet (UV) radiation is needed to produce oxidizing species with high enough Nernst potentials to oxidize silver.8
One-month silver corrosion results from Phase II for the continental United States are reported in Figure 3. A horizontal bar chart (left) reports the speciated charge measured between silver chloride (AgCl) reduction and silver sulfide (Ag2S) reduction and the total charge.
A color-indexed bubble chart of sites overlaid on a map of the United States (right) is included where the size of each bubble corresponds to the total reduction charge measured during galvanostatic reduction analysis (which, in turn correlates to the amount of silver products on the surface). The color of each bubble corresponds to the fraction of AgCl vs. Ag2S on the silver surface.
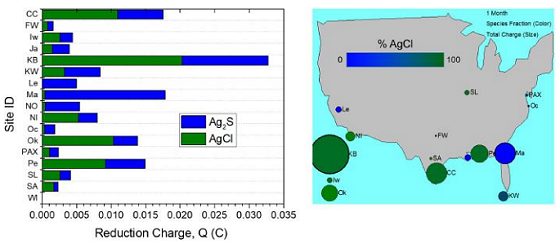
Most sites contain a mix of AgCl and Ag2S; however, a few sites were almost entirely composed of Ag2S (Lemoore, Mayport, and New Orleans). Ag2S is the most stable of the silver compounds, though AgCl is also very stable.
There is a noticeable preference for silver oxidation for the southern sites, with the top six sites being located below the 31st parallel. However, this is not the only factor, as other low-ranking sites also fall below this threshold.
Conclusions
• Steel mass loss varied across the 17 sites. Typically, high mass loss was observed for coastal sites, but not definitively. Some results were unexpected initially, like WI being the most severe site (this is actually consistent with anecdotal evidence from WI maintainers), despite being a cool, low UV site located in a relatively sheltered bay. This finding supports on-going efforts to re-evaluate current maintenance intervals based on updated ESI data.
• Silver reduction analysis indicates that the ambient environment between the various sites is different in terms of quantity and type of deposited species. This data should be used to improve the accelerated test conditions used to mimic long-term testing at the different sites.
• Important differences in corrosion behavior between the 17 sites are observed. The data generated under this program will be used to adapt the NRL Key West test site to mimic different local conditions (using salt spray, length of exposure, or freshwater rinsing).
• These preliminary results will be supplemented by further analysis of returning samples and collected meteorological data to develop a more comprehensive understanding of the factors that control atmospheric corrosion.
Acknowledgements
Research was performed in cooperation with the Naval Air Warfare Center Aircraft Division.
Source: U.S. Naval Research Laboratory, www.nrl.navy.mil.
References and About the Authors