NACE International member Frank Ansuini wrote a Materials Performance article about his 2007 visit to Antarctica,1 noting that most of it “… is considered a desert by climatologists, and a truly magical place by those fortunate enough to visit it.” Almost 70% of earth’s fresh water is located in ice sheets, glaciers, and permanent snow,2 and ~89% of that is contained in the Antarctic ice sheet.
Take a moment to reflect on the fact that a little over 60% of the world’s fresh water is frozen in a desert. However, even along the coast, where corrosive aerosol particulates are present from the stormy seas, corrosion of metals proceeds at a slow rate. To control corrosion of metals, one important requirement is an understanding of the environment. When thinking of this, we should include the macro, meso, and microenvironments.3 Figure 1 shows possible macro, meso, and microenvironments for atmospheric exposure on the Antarctic Peninsula.
The macro environment includes such things as temperature, moisture (precipitation, relative humidity [RH], condensation/ dew), and wind. Several possible elements are listed for both meso and microenvironments, but those lists could easily be modified or extended to fully describe the environment at a specific location. It is appropriate then to become familiar with all the factors your corrosion situation may involve. One-size-fits-all solutions are not always successful.
Exposure—Conditions Really Matter
Natural Environment Corrosion
To control corrosion, it is helpful to categorize the environment. We differentiate atmospheric corrosion from underground corrosion. We also differentiate corrosion that occurs on submerged metals from underground corrosion. Further still, we differentiate submerged exposure to freshwater from that occurring in saltwater. Clearly identifying pertinent exposure conditions helps corrosion engineers and technicians to provide the best corrosion solution that the service life and budget allow.
Macro Environment
The macro-level describes the general environment or largest setting that may have a significant impact to corrosion. The Earth, for some evaluations, might be the macro environment but typically that scale is not relevant to corrosion. When considering atmospheric corrosion, the macro environment might be a city, county, or province.
Although not specific to corrosion, on the macro level the state of Alaska (USA) requires architects, engineers, and land surveyors to successfully complete an approved course in arctic/cold regions engineering. This provincial action is because portions of the state of Alaska extend north of the Arctic Circle and proper engineering solutions may likely require knowledge of how very cold conditions can impact successful designs.
Temperature: As a rule of thumb, the Arrhenius Equation states that for most chemical and biological reactions, a 10 °C temperature increase roughly doubles the corrosion rate.
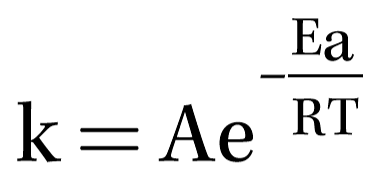
The average temperature for Tampa, Florida, USA is about 22 °C, while that for Vancouver, British Columbia, Canada is 19.5 °C. These two cities are both located on marine coasts. With a little over 10 °C difference in temperature, Arrhenius predicts the service life of similarly hot-dip galvanized highway signs and guardrails would be about twice as long in Vancouver as those in Tampa, and from discussions with transportation engineers in both locales that is about the actual case. Understand, being a rule of thumb, this twofold rate modification from a 10 °C temperature change does not apply to all corrosion situations. However, it is reasonable that equipment corrosion will be reduced in Antarctica, where the average temperature is often less than where Celsius and Fahrenheit meet at -40 °C/F.
Moisture: Moisture includes precipitation, dew, and condensation, where the latter two are impacted by RH. Moisture is necessary for most corrosion reactions. As with temperature, another rule of thumb is that a wet atmospheric environment is more corrosive than a dry environment. Since Antarctica is, on average, the coldest and driest continent, you could double up moisture with temperature and should reasonably expect the two combined to reduce corrosion.
Pollutants: In coastal areas, the atmosphere often includes freshly-dried sea particulates that have a high chloride ion content. When aerosol chloride-bearing particulates settle on metal surfaces they most often aggravate corrosion. The atmosphere in industrial and urban areas can also contain other corrosive aerosol pollutants. The Antarctic also has other aerosol pollutants, but it comes from the penguins and not smokestacks.
Meso Environment
The meso environment rests between the macro and the microenvironments. As shown in Figure 1, this includes factors, such as how close is it to the shoreline, prevailing wind and wave action, whether it is boldly exposed or not, and if coatings are present. It could also include the presence and number of wildlife in the immediate proximity.
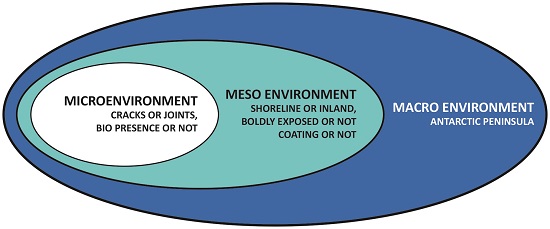
Microenvironment
In the microenvironment, we are considering smaller, localized factors. Are cracks or joints present where capillary suction can draw water in allowing the aqueous chemistry to change, such as with chloride contamination allowing the local pH to be reduced? Another factor might be localized stresses. Finally, is there biological activity occurring on the surface of the metal? This could be growth on the metal surface or contamination by aerosol biological material carried on the wind.
The Environment May Change
Environments can change over time. Such changes may generate further changes. Following are several cases observed along the Antarctic Peninsula.
Vernadsky Research Base
The Vernadsky Research Base (Figure 2, top) is located on Galindez Island in the Argentine Islands off the Antarctic Peninsula. The base has been operated by the National Antarctic Scientific Center of Ukraine since 1996. From 1947 to 1996, the base was operated by the United Kingdom as Faraday Station, named after Michael Faraday, the British scientist and a corrosion science founding father. In just two years they will be celebrating its 75th anniversary as a research station.
Penguins
It is well known that one aspect of visiting penguin colonies is the aroma from their guano. Galindez Island is a desert location, so there is little precipitation to wash the guano from nesting sites. However, the desert air quickly dries the penguin feces and the wind carries away the fecal dust.
Algae Produces Rusty Watermelon Snow
When the guano dust settles on ice or snow, it fertilizes the algae
Chlamydomonas nivalis, increasing population growth. The snow algae are greenish in color during the reproductive stage. The color turns a reddish color when the algae are dormant—think of it as autumn arriving in Antarctica.

The gentoo penguin population at Vernadsky Station (Figure 3) increased 345% from 2011 to 2018, while a larger colony located about 1 km away increased 305% over the same time period.4 More penguins means more fertilizer and greater algae growth. Figure 4 shows not rust staining, but algae colored watermelon snow.
Both the green and red coloring of the algae reduce the snow and ice albedo—a measure of Earth surface reflectivity. Sea ice reflects 50 to 70% of incoming sunlight; sea ice with snow cover can reflect almost 90%; the colored algae absorb more sunlight that 1) melts ice locally to provide water for the algae to better grow, 2) it increases the overall melting of ice more readily than other light-absorbing impurities like dust or black carbon.5 So penguins can impact the environment as well as people.

Antarctic Corrosion
When it comes to corrosion, Antarctica being a polar desert clearly helps to reduce the rate. That said, the majority of research stations are located on the coast where aerosol contamination from seawater and the penguins might aggravate issues.
 Figure 5 shows some barrels located at the British Base W located on Detaille Island south of the Antarctic Circle and abandoned in 1957. Although corrosion can clearly be seen, the barrels look to be in very good shape after 63 years of close exposure to a windy and stormy sea. The barrels were galvanized, so that coating provided very good service considering the age of the barrels.
Figure 5 shows some barrels located at the British Base W located on Detaille Island south of the Antarctic Circle and abandoned in 1957. Although corrosion can clearly be seen, the barrels look to be in very good shape after 63 years of close exposure to a windy and stormy sea. The barrels were galvanized, so that coating provided very good service considering the age of the barrels.
Figure 6 shows some corrosion on a radio key and headphone, to the rear a microammeter, and to the right the side of a radio amplifier. The radio set is also located in a building at Base W, which was made a United Kingdom Heritage Site in 2009. In 2010, after over 50 years of uncontrolled decay, the base was made structurally secure and weather tight. Given that extended exposure, the equipment corrosion seems quite modest.
Active bases, like Vernadsky, do get some maintenance, like coating, done on the few summer days when the weather allows. Figure 2 shows the water tank, which is visibly in good shape externally. I have personal experience with water tank ice cake formation in northern tier states and Alaska. Perhaps we can address those issues in another article.
British Base J is located on Prospect Point in Graham Land of the Antarctic mainland. Base J was only occupied from 1957 to 1959. Per international agreements, the station, save the concrete footers, was removed in 2004. Figure 7 shows some of the remaining footers and a close-up of a galvanized rod used to anchor the previous building to the footer. Note that much of the galvanizing is still intact, yet the location is very close to the ocean.

Conclusions
The environmental impacts must be considered as part of any design process. Early in the design, the macro, meso, and microenvironments should be defined, and their various impacts evaluated. Potential changes and their impacts should be considered. From a brief visit, it appears that along the Antarctic Peninsula the low temperature and dryness retard corrosion in the face of corrosion aggravating marine conditions. The Antarctic is a very amazing place and if someone offers you the opportunity to visit, immediately embrace your good fortune and go.

References
1. F. Ansuini, “Corrosion of Old Whaling Station Processing Equipment in Antarctica,” MP 46, 4 (2007): pp. 56-57.
2. I. Shiklomanov, “World freshwater resources,” in Water in Crisis: A Guide to the World’s Fresh Water Resources, ed. P.H. Glieck (New York, NY: Oxford University Press, 1993).
3. J. Tinnea, “Specialist Examination & Diagnosis,” Plenary Lecture, Brian Cherry International Concrete Symposium, held July 26-27, 2017 (Brisbane, Australia: Australasian Corrosion Association, 2017).
4. V.M. Smagol, D.V. Pilipenko, A.O. Dzhulai, “Dynamics of Numbers and Occurrence of Colonial Species of Birds in the Area of Ukrainian Antarctic Station Akaemik Vernadsky,” Vestnik Zoologii 53, 6 (2019): pp. 491–500.
5. M. Stibal, et. al, “Algae Drive Enhanced Darkening of Bare Ice on the Greenland Ice Sheet,” Geophysical Research Letters (2017).