Ammonia flue gas desulfurization (AFGD) has attracted increasing attention to control sulfur dioxide (SO2) emissions. Due to its high desulfurization efficiency (95 to 99%), no secondary pollution, and the ammonium sulfate [(NH4)2SO4] byproduct being a fertilizer, AFGD has started to become adopted by more and more companies, especially with new and stricter environmental regulations.1-2 However, serious corrosion can occur, especially pitting corrosion, which is due to the acidic ammonium sulfate slurry containing corrosive ions, raising serious concerns over employing AFGD in normal operations.3-4
 Therefore, in this article, seven metal materials, including Q235 steel (Grade D), Types 304 and 316 stainless steel (SS) (UNS S30400 and UNS S31600), titanium alloy (TA0), 2205 duplex SS (S32205), 2507 super duplex SS (UNS S32750), and Hastelloy C-276 (UNS N10276), which were the most widely used in AFGD systems, were placed in a custom-made device containing AFGD slurry for a pitting corrosion test. This research can provide the technical foundation for the further materials selection and application of AFGD systems.
Therefore, in this article, seven metal materials, including Q235 steel (Grade D), Types 304 and 316 stainless steel (SS) (UNS S30400 and UNS S31600), titanium alloy (TA0), 2205 duplex SS (S32205), 2507 super duplex SS (UNS S32750), and Hastelloy C-276 (UNS N10276), which were the most widely used in AFGD systems, were placed in a custom-made device containing AFGD slurry for a pitting corrosion test. This research can provide the technical foundation for the further materials selection and application of AFGD systems.
Experimental Procedure of Pitting Test
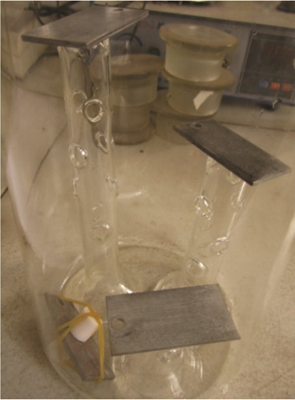
The AFGD slurry included ammonium sulfate (5% solid content), chloride ions (20,000 mg/L), and fluoride ions (5,000 mg/L). A glass beaker was custom made as the test container, as shown in Figure 1. Magnifying the hollow glass holder shows that the metal coupon can be stable on the tops of points S, W, and T for the pitting corrosion test (Figure 2).
The pretreatment and post-treatment of the coupon are the same as the method of weight loss.5 After that, the coupon was placed on the top of the holder in the beaker, and the simulated AFGD slurry was poured into the beaker slowly. After the heating and reflux device was assembled, the beaker was moved into an electro-thermostatic water bath with the lid on for the test. The baths were kept at 65 °C for 60 days. Periodically, the coupons were taken out for observation and reweighing. The holders were designed with three different heights as parallel experiments.

Precautions were taken to include the following. The amount of slurry per square centimeter of the coupon must be more than 20 mL. The pitting corrosion device must ensure that all the coupons were in the slurry and cannot touch the beaker. Holes in the holder tubes were needed to maintain the solution convection. In principle, there should be only one coupon in a beaker, but for the coupons with the same steel grade and heat treatment, if they meet other experiment conditions, more than one coupon was placed in the same beaker; however, the coupons did not touch each other.6
Results and Discussion
Pitting corrosion of seven metal materials, including Q235, Type 304 SS, Type 316 SS, a titanium alloy, 2205, 2507, and Hastelloy, were tested in AFGD slurry in the laboratory for two months, and the results are shown in Figures 3 through 6.
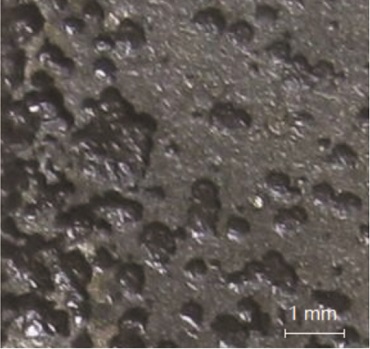 Figure 3 shows severe pitting corrosion on the Q235 steel specimen. Ammonium sulfate is a strong acid-weak alkali salt that makes desulfurization slurry acidic and the corrosion of carbon steel (CS) is extremely serious due to the hydrogen depolarization in this environment.7
Figure 3 shows severe pitting corrosion on the Q235 steel specimen. Ammonium sulfate is a strong acid-weak alkali salt that makes desulfurization slurry acidic and the corrosion of carbon steel (CS) is extremely serious due to the hydrogen depolarization in this environment.7
The research also focused on Types 304 and 316 SS, which are used for most connectors and pumps in AFGD systems. Pitting corrosion was observed on both Types 304 and 316 SS (Figures 4 and 5), especially on the Type 304 SS, where the pitting corrosion was severe and penetrated the coupon thickness. The AFGD system is a special closed system, where only the ammonium sulfate product and water vapor are effectively discharged from the system, while all other water-soluble substances continue to circulate. So, Types 304 and 316 SS suffered serious corrosion related to the continuous cycle and, over time, accumulated large amounts of chlorine and fluoride ions in the AFGD system.8-9
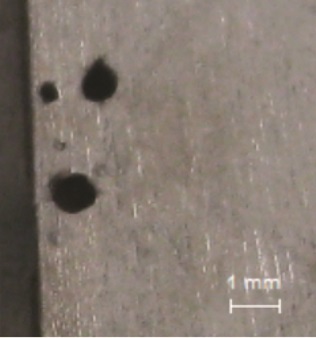 Pure titanium, TA0, shows strong corrosion resistance in many environments due to being a metal that readily self-passivates. In an oxygen-containing medium, a dense and strong adhesive oxide film typically is generated on the surface of titanium, which protects the substrate from corrosion. Even if the passive film was damaged by particle abrasion or other reasons, it can be healed or regenerated very quickly in the presence of oxygen. However, the severe pitting corrosion was observed on the titanium alloy surface in the AFGD slurry as shown in Figure 6. As previously mentioned, chlorine and fluoride ions accumulate as a result of the recycling of the ammonium sulfate slurry. Chloride ions likely played a significant role in the local corrosion for the film and can cause pitting and perforation. However, the most dangerous substance is the fluoride ion that will quickly destroy the oxide film on the titanium surface under the acidic conditions. Furthermore, with titanium this damage cannot be repaired.10
Pure titanium, TA0, shows strong corrosion resistance in many environments due to being a metal that readily self-passivates. In an oxygen-containing medium, a dense and strong adhesive oxide film typically is generated on the surface of titanium, which protects the substrate from corrosion. Even if the passive film was damaged by particle abrasion or other reasons, it can be healed or regenerated very quickly in the presence of oxygen. However, the severe pitting corrosion was observed on the titanium alloy surface in the AFGD slurry as shown in Figure 6. As previously mentioned, chlorine and fluoride ions accumulate as a result of the recycling of the ammonium sulfate slurry. Chloride ions likely played a significant role in the local corrosion for the film and can cause pitting and perforation. However, the most dangerous substance is the fluoride ion that will quickly destroy the oxide film on the titanium surface under the acidic conditions. Furthermore, with titanium this damage cannot be repaired.10

No pits were observed on the 2205, 2507, and Hastelloy C-276 specimens. This is mainly because the 2205 and 2507 duplex SS have the advantages of both ferrite and austenitic SS, and Hastelloy C-276 contains a great deal of nickel and chromium. So, they have excellent resistance to both pitting corrosion and uniform corrosion.

Conclusions
Q235 CS, Types 304 and 316 SS, and titanium TA0 alloy readily suffered pitting corrosion in AFGD slurry due to the continuous cycle producing an accumulation of large amounts of chloride and fluoride ions under the acidic conditions. Therefore, Q235, Types 304 and 316 SS, and titanium alloy TA0 were not suitable for AFGD systems directly, unless special anti-corrosion treatments were used, like the use of inhibitors. No pits were observed on the 2205, 2507, and Hastelloy C-276 specimens after six months, and thus, they can be considered as equipment material, especially for critical equipment.
References
1 L.M. Zhou, et al., “Process Optimization for Ammonia-Based Flue Gas Desulfurization,” Chemical Engineering 42, 4 (2014): pp. 7-12.
2 Y.Y. He, “Flue Gas Ammonia Desulfurization Technology and Its Development,” Environment Protection of Chemical Industry 32, 2 (2012): pp. 141-144.
3 V.A. Bochicchio, “FRP Chimney Liners for Power Plant Flue Gas Desulfurization,” MP 50, 9 (2011): pp. 42-46.
4 Z.Y. Lian, et al., “Corrosion Investigation of Ammonia Flue Gas Desulfurization,” MP 55, 9 (2016): pp. 50-53.
5 Z.Y. Lian, et al., “Anticorrosion Performance and Field Application of a New Rebar Inhibitor,” Applied Mechanics and Materials 310 (2013): pp. 95-100.
6 GB/T 17897-1999, “Test of Pitting Corrosion Resistance of Stainless Steels in the Ferric Chloride Solution” (Beijing, China: SAC, 1999).V
7 S.D. Wang, et al., “Study of N-1L Corrosion of Carbon Steel in Acidic Desulfurizer Performance,” Applied Mechanics and Materials 670 (2014): pp. 37-40.
8 I. Martinovic, et al., “Electrochemical Behaviour of Stainless Steel in Acidic Fluoride Media,” International Journal of Materials Research 106, 10 (2015): pp. 1,067-1,076.
9 M. Li, et al., “Electrochemical Corrosion Behaviour of Type 316 Stainless Steel in Acid Media Containing Fluoride Ions,” British Corrosion Journal 36, 3 (2001): pp. 179-183.
10 L. Yang, et al., “Degradation Behavior of Ti-6Al-4V Alloys for Dental Applications in Acidic Artificial Saliva Containing Fluoride Ion,” Journal of Wuhan University of Technology-Materials Science Edition 32, 4 (2017): pp. 926-934.