Whether fueled by coal, gas, nuclear power, or geothermal energy, these types of power plants have one common feature: they use heat to generate electricity. Geothermal power plants use geothermal resources—reservoirs of hot water that exist at varying temperatures and depths below the Earth’s surface—as a heat source.
In the geothermal energy production process, heat from the Earth is accessed by drilling water or steam wells in a process similar to drilling for oil. A production well, which can be more than a mile (1.6 km) deep, is drilled into a known geothermal reservoir. The hot geothermal fluids then flow through pipes to a power plant where the hot, pressurized geothermal fluid or a secondary working fluid is allowed to expand rapidly and provide rotational or mechanical energy to turn the turbine blades on a shaft for electricity generation. Typically, an injection well is also drilled to return used geothermal fluids to the geothermal reservoir.
According to NACE International members W.D. MacDonald and J.S. Grauman with Titanium Metals Corp. (Exton, Pennsylvania, USA), tapping the energy potential in high-power geothermal wells can be restricted by the ability to specify materials that will withstand the extreme environmental conditions. Geothermal brine reservoirs typically pose severe corrosion problems for operators—due to the combination of steam, water, and brine at elevated temperatures; the presence of hydrogen sulfide (H2S) and carbon dioxide (CO2); and high chloride content—yet these resources can also yield high-enthalpy steam that produces higher power outputs, so they have become very desirable fields to develop.1
The Salton Sea Known Geothermal Resource Area (KGRA) is a high-enthalpy geothermal field in the Salton Sea, the largest lake in California, USA, and saltier than the Pacific Ocean. Located ~60 miles east of San Diego, California, the area contains one of the world’s highest-temperature resources for geothermal energy.2 The temperature of the hypersaline brine associated with the field is typically greater than 250 °C, which facilitates a very efficient flashed steam system. According to the U.S. Department of Energy, the Salton Sea KGRA is home to the Imperial Valley Geothermal project, which consists of 10 generating plants with a combined net capacity of ~327 MW.3
MacDonald and Grauman comment that in the Salton Sea KGRA, corrosion of casing and equipment fabricated of conventional alloys was unacceptably high and economically intolerable due to the high chloride content, low pH, and corrosive non-condensable gases contained in the brine. Because exposing possible corrosion-resistant alloy casing materials to actual operating environments is challenging and expensive, end users have relied on laboratory experiments to prequalify materials.
Since the early 1990s, titanium has been used in the Salton Sea KGRA to control the excessive corrosion experienced in production wells. Early corrosion testing in the geothermal brine, however, showed that unalloyed titanium was prone to localized attack under the most severe conditions in these brines. Some of the more corrosion-resistant titanium alloys, however, appeared to resist any type of corrosion attack. MacDonald and Grauman note that these results correlate well with other chloride media testing that show unalloyed titanium can be susceptible to crevice corrosion attack in seawater and other concentrated brines at temperatures exceeding 80 to 90 °C, but more corrosion-resistant titanium alloys can withstand corrosion attack in these same brines at temperatures greater than 315 °C.
Over the last 30-plus years, corrosion testing of more than 10 different titanium alloys in actual and simulated hypersaline geothermal brines has tracked mass loss, crevice, pitting, and stress corrosion cracking behavior of these materials. Test conditions included brines with a composition typical of the Salton Sea geothermal brine—pH usually adjusted to create an acidic media in the range of 4 to 6; the presence of chloride (140,000 ppm), sodium (53,000 ppm), calcium (28,800 ppm), and potassium (16,500 ppm); partial pressures of corrosive gases such as CO2 and H2S; and temperatures up to 304 °C. The outcome of these geothermal exposure tests, both in the field and in numerous laboratory exposures, showed that titanium always ranked at or near the top in material exposure performance.
In their paper presented at CORROSION 2018,1 MacDonald and Grauman discuss laboratory corrosion testing of UNS R53400 and UNS R56404 titanium alloys, as well as UNS N06625 nickel alloy, in a simulated hypersaline geothermal system such as the one found in the Salton Sea KGRA.
 The corrosion testing was performed at a commercial laboratory and used specimens supplied by MacDonald and Grauman. The laboratory machined the specimens to unloaded dimensions of 100 by 9 by 3 mm and then loaded them according to ASTM G304 by bending them 180-degrees into U-bends with UNS N10276 nickel alloy bolts. Ceramic shoulder washers were used to electrically isolate the bolts from the specimens. MacDonald and Grauman provided the desired gas and liquid compositions via stream reports, and this information was used to develop the test brine’s desired gas and liquid compositions (see Table 1). A thermodynamic modeling software package was used to predict the concentrations of H2S and CO2 required to achieve the target partial pressures and pH at the test temperature (304 °C).
The corrosion testing was performed at a commercial laboratory and used specimens supplied by MacDonald and Grauman. The laboratory machined the specimens to unloaded dimensions of 100 by 9 by 3 mm and then loaded them according to ASTM G304 by bending them 180-degrees into U-bends with UNS N10276 nickel alloy bolts. Ceramic shoulder washers were used to electrically isolate the bolts from the specimens. MacDonald and Grauman provided the desired gas and liquid compositions via stream reports, and this information was used to develop the test brine’s desired gas and liquid compositions (see Table 1). A thermodynamic modeling software package was used to predict the concentrations of H2S and CO2 required to achieve the target partial pressures and pH at the test temperature (304 °C).
The brine was mixed at ambient conditions and then bubbled with CO2. MacDonald and Grauman note that the solution turned brown as it was mixed, which was most likely caused by oxidation of iron ions from the ferrous chloride (FeCl2). The deaerated solution was added to a deaerated autoclave containing the stressed specimens (duplicate specimens of each alloy material were tested in the same vessel), and the H2S and CO2 were added separately by liquid loading. The vessel was then sealed and the temperature increased. The 30-day exposure period began when the temperature reached 304 °C. After testing, the specimens were cleaned in an ultrasonic bath with a warm phosphate detergent solution followed by a warmed and inhibited hydrochloric acid (HCl) solution and light scrubbing with a pig-bristle brush. Adherent black crystals remained on the UNS R53400 titanium alloy.
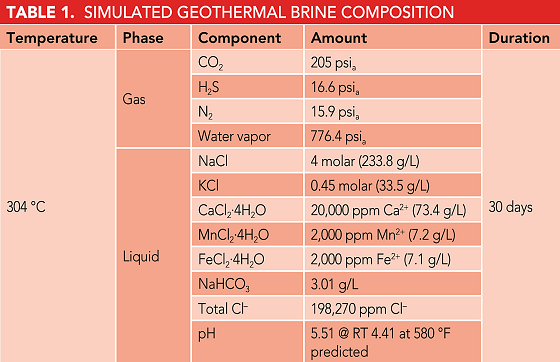
Preliminary examination of all samples at 20X magnification (per ASTM G30) showed no obvious cracking or pitting on the apex of the U-bend (Figure 1). A thorough examination of the inside and outside diameter of each sample was completed at 20X to 40X magnification. Small shiny areas noted on the outside diameter of Sample 1 of the UNS N06625 nickel alloy were selected for further analysis using a high-magnification, three-dimensional microscope. The analysis confirmed these shiny areas were shallow corrosion pits on the surface of the sample. Shiny spots also were present on the inside diameter of the sample, but could not be further examined or photographed without sectioning the sample.
A relatively larger and deeper shiny area on Sample 2 of the UNS N06625 nickel alloy also was a major area of interest. This spot was located in a crevice covered by the ceramic washer. No grind marks were visible in the affected area when it was analyzed with the high-magnification microscope, which confirmed that the spot was crevice corrosion.
While no shiny corrosion spots were observed on the surfaces of the UNS R53400 titanium alloy U-bend samples, several small, darkened areas were seen beneath the ceramic washer. Clearly visible, uninterrupted grind marks were seen in this area, and light scratching removed the dark surface film to reveal unaffected metal underneath. The film appeared to be a surface deposit, but was not attributed to any type of corrosion. Titanium corrosion deposits, which are known to be extremely tenacious, would not be as easily removed as this deposit.

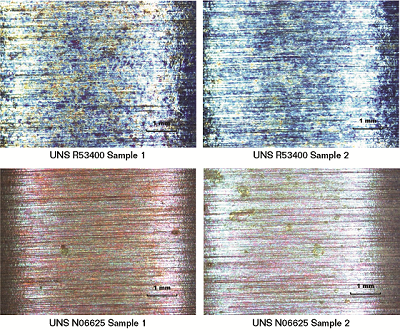
As a final check for crack susceptibility, U-bend samples of the UNS N06625 and UNS R53400 alloys were slowly stressed to increase the bend tension by 50% (i.e., the distance between the ends of the samples was reduced from 36 mm to 18 mm) to determine if any evidence of crack initiation could be observed on the apex of the sample surfaces. Analysis of the surface of both the UNS N06625 and UNS R53400 samples after the 50% bend increase showed no cracking. Figures 2 and 3 show the apex of the UNS N06625 and R53400 at different magnifications, respectively.
From the experiment, MacDonald and Grauman summarized that the initial surface examination showed no cracks on the UNS N06625 nickel alloy and UNS R53400 titanium alloy samples after exposure to the high-temperature brine, but did reveal small surface features for each. UNS N06625 nickel alloy U-bend samples exhibited both crevice corrosion at the metal-washer area and also shallow pitting on the surface of each of the two samples, although the corrosion was very slight. Discolored areas observed at the metal/washer area of a UNS R53400 titanium alloy sample appeared to be an easily removed deposit. No corrosion was observed under these deposits. Applying further stress on U-bend samples did not lead to any type of crack on any of the alloy samples.
According to MacDonald and Grauman, titanium’s resistance to hot reducing chloride environments makes it an appropriate material for use in geothermal service, and they foresee the continued use of titanium as the Salton Sea KGRA is further utilized as a geothermal energy source. Future use of titanium also could possibly be seen in low-chloride geothermal steam and water service where corrosion occurs from condensed acid or non-condensable acid gases.
Titanium is also being considered and tested for use in more typical steam generating wells where HCl condensation has proven to be a corrosion issue, as well as enhanced geothermal systems where recirculated water from surface-injected fluid becomes increasingly more corrosive over time.
Bibliography
Office of Energy Efficiency & Renewable Energy. “Geothermal Basics.” https://www.energy.gov/eere/geothermal/geothermal-basics. October 12, 2018.
References
1 W.D. MacDonald, J.S. Grauman, “Exposure Testing of UNS R53400, R56404 and N06625 in Simulated Salton Sea Geothermal Brine,” CORROSION 2018 paper no. 11547 (Houston, TX: NACE International, 2018).
2 Office of Energy Efficiency & Renewable Energy, “Salton Sea Power Plant Recognized as Most Innovative Geothermal Project,” https://www.energy.gov/eere/geothermal/articles/salton-sea-power-plant-recognized-most-innovative-geothermal-project (October 12, 2018).
3 Office of Energy Efficiency & Renewable Energy, “Imperial Valley Geothermal Area,” https://www.energy.gov/eere/geothermal/imperial-valley-geothermal-area (October 12, 2018).
4 ASTM G30-97(2016), “Standard Practice for Making and Using U-Bend Stress-Corrosion Test Specimens” (West Conshohocken, PA: ASTM, 2016).