Back in 1943, NACE International was established in Houston, Texas, USA by 11 engineers focused on cathodic protection (CP) to address metal pipeline degradation. Now 36,000+ members strong, NACE has evolved into a worldwide organization that is involved in every industry and area of corrosion prevention and control. This year, NACE has been celebrating its 75th anniversary, a milestone made possible by the knowledge, expertise, and continued support of its members from around the globe. After honoring NACE’s history over the last year with classic MP articles from the past, guest editorials from NACE Past Presidents and Fellows, content about NACE’s evolution across all activities, and more, our latest issue is dedicated to the future of corrosion control as seen by experts in the field. In this roundtable special, panelists share their predictions on where the corrosion industry is going in the next 25 years and beyond.
For Part 1 of this series, the panelists are Nick Birbilis, FNACE, deputy dean of the College of Engineering and Computer Science, Australian National University (Acton, ACT, Australia); and Rick Eckert, senior principal engineer, corrosion management, at DNV GL (Dublin, Ohio, USA).
Nick Birbilis
When considering the important future developments in the industry of corrosion control, the prospects are as complex as they are plentiful. Identifying such prospects requires one to zoom out a little from the technological question (i.e., what will the future of corrosion control resemble?) and consider the key factors or indicators that can be rationally identified as significant in influencing the future of corrosion control. Such factors include, but are not limited to, the following.
Legislation
Undoubtedly the unpredictability of politics is something we are constantly reminded of. In many cases, the most (financially and socially) significant decisions when it comes to corrosion control are often placed in the hands of lawmakers. Two significant examples include the phasing out and imminent replacement of chromate-containing corrosion preventative compounds,1 and the long-term disposal plans for nuclear waste. In the case of chromate replacement, some nations and industries are somewhat more advanced than others; however, it is fair to say that no equivalent (and broadly applicable) alternatives have been found to date—such that the corrosion protection regimes we will see for everything from galvanized garden sheds to the next commercial jetliner are yet to be determined (let alone their long-term durability). The issue of nuclear waste storage varies from nation to nation for countries with nuclear power generation; however, the world is watching for a long-term strategy in the United States, which is yet to be determined following the shelving of the Obama-era Yucca Mountain Repository project.
New Alloys/Materials
The development of new materials is now occurring at a pace greater than ever before. In part, computation has allowed materials design to evolve from what was historically plant trials to documented demonstrations of desktop alloy design with industrial utility.2 Alloy development has come so far since the Second World War that a catchphrase of the automotive industry is “nearly all the alloys used in an automobile are different each 10 years,” meaning that materials we seek to protect are also always evolving. In fact, even in what is considered a very conservative industry—the aircraft industry—the change in the dominant structural alloy of commercial aircraft has also seen an active evolution from the aluminium alloys AA7022, to AA7079, to AA7075, to AA7050, to AA7150, to AA2050—all in the past five decades alone. This latter example relating to the evolution of aircraft alloys is an example of changing the alloy used in order to improve durability (i.e., a decision based on corrosion protection, albeit corrosion resistance inherent to the alloy). In such a vein, the design of corrosion-resistant alloys is an area of active research.
A “hot topic” at the moment is so-called compositionally complex alloys (a subset of which are often termed high-entropy alloys) that have demonstrated exceptional corrosion resistance in aqueous and atmospheric conditions.3 Such alloys are not yet optimized in terms of a complete property portfolio for engineering applications, but there is no doubt that future corrosion protection will be dealing with (i) new materials that are presently under development, and (ii) materials with inherent corrosion resistance being designed to be more durable—and not necessitating “traditional” corrosion control. I could add many more examples, but we can look no further from the present rapid uptake of additive manufacturing to produce netshape components, from a range of new (and old) alloys, with disabilities that are only presently under study.
Complex Systems
The great unknown is the evolution of complex systems. If we went back 15 years (or less), most of us were not carrying around a laptop, let alone a smartphone. Yet now the pervasive nature of new technology sees us all carrying items that are being used in a manner (and in environments) for which such materials have not previously been used. In other words, as technologies evolve (in general), it’s very likely we will see more drones, more driverless cars, and then a transition to perhaps flying cars. I paint this picture to emphasize that a flying car would obviously need to be light and have a unique (cost-effective) propulsion system, as we can’t all afford a superalloy gas turbine. As such, we don't know precisely what we will be dealing with, but one certainty is that there will be many new materials and technology interruptions, and all will have ramifications in terms of corrosion control.
In regards to complex systems, there are numerous ones that are also presenting the extremes of our capabilities in corrosion protection. For example, the sequestration and transportation of supercritical carbon dioxide (CO2) (in the carbon capture and storage cycle) remains a significant challenge in the case of contaminated CO2 whilst the renewable energy sector (which is not only coming but will be dominant in the next 25 years by all projections) presents durability unknowns in everything from solar thermal generation to proposed grid storage solutions.
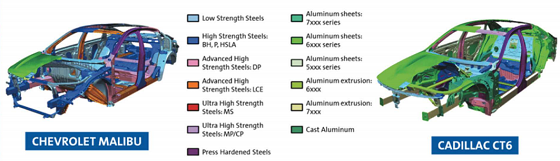
Finally, I will also provide one example that combines both issues of legislation and complex systems, highlighting the complexity of future corrosion control. In most nations, the United States being no exception, automotive emission policy (of which the state of California has amongst the world’s strictest targets) means that lightweight material systems are now being integrated into automobiles. A recent study (2018) by Liu and co-workers4 of General Motors reveals the extreme complexity of a contemporary mass-market automotive “body in white” (Figure 1), indicating that the durability of an automobile relies on the durability of a multi-material system with widely varying material types (and electrochemical personalities).
In summary, one thing that we should always remember, especially all of us corrosion engineers (a.k.a., “rust busters”), is that engineering materials are all “anthropogenic”—in other words, man-made. As a result, their properties, good or bad, are our doing. Consequently, we have the ability to create materials with durability in mind and an increasing responsibility to do so on the basis of the planet’s finite resources. In the future, for corrosion control, we need to be smarter! We also need to learn more from the past and be more proactive in education. One alarming point that was raised from the most recent of the rotating national surveys on the cost of corrosion, the latest being recently published from a meticulous national survey in China,5 is that the percent GDP cost of corrosion is not dropping; this can only mean that society is not learning, or is willing to make errors in judgment. Assuming it is not the latter, there is an increasingly significant role for NACE International in the future of corrosion control.
Richard B. Eckert
 Microbiologically influenced corrosion (MIC) impacts many different assets and industries, and yet it is a corrosion process that is still not completely understood despite the current advances being made in the field of genomics. MIC is typically found to be associated with diverse functional types and genera of microorganisms that develop into biofilms, forming syntrophic or complementary metabolic relationships that enhance microbial growth and activity. The spatial and metabolic relationships between the different members of the biofilm community and the electrochemical process of corrosion are still being investigated. The application of molecular microbiological methods (MMM) in the oil and gas industry has led to a greater understanding of the diversity of bacteria and archaea (and fungi) that exist in production and storage wells, piping, process plants, and tanks; however, characterizing a multitude of different microorganisms has not always been helpful to asset operators who simply want to know how to mitigate MIC. The industry wants a straightforward diagnostic test for MIC that provides actionable results. Genomic methods fall into different “omics” scientific disciplines, including:
Microbiologically influenced corrosion (MIC) impacts many different assets and industries, and yet it is a corrosion process that is still not completely understood despite the current advances being made in the field of genomics. MIC is typically found to be associated with diverse functional types and genera of microorganisms that develop into biofilms, forming syntrophic or complementary metabolic relationships that enhance microbial growth and activity. The spatial and metabolic relationships between the different members of the biofilm community and the electrochemical process of corrosion are still being investigated. The application of molecular microbiological methods (MMM) in the oil and gas industry has led to a greater understanding of the diversity of bacteria and archaea (and fungi) that exist in production and storage wells, piping, process plants, and tanks; however, characterizing a multitude of different microorganisms has not always been helpful to asset operators who simply want to know how to mitigate MIC. The industry wants a straightforward diagnostic test for MIC that provides actionable results. Genomic methods fall into different “omics” scientific disciplines, including:
• Metagenomics—the study of genetic material (DNA) from entire microbiological communities in a given environment to understand diversity and function
• Proteomics—the study of proteins as a measure of gene expression and cellular activities and functions
• Metabolomics—the comprehensive study of chemical metabolites produced by microbiological communities to help characterize their activities
Each of these “omics” produces information that needs to be translated and integrated with other information about the chemical environment and physical conditions in which the collective of microorganisms live in order to understand who is there and what they are doing, particularly in relation to corrosion. Since, to date, there has been no singular data element found that is diagnostic for MIC, a successful future test method would likely need to integrate numerous chemical and microbiological factors using a model and some form of machine learning, based on a large and reliable data set. From such a future model and data set, relationships between the microbiology, chemistry, materials science, and physical conditions of a given environment could be determined and the propensity for MIC positively identified.
Probabilistic modeling tools may be one way to start predicting MIC based on the information available today; in the future, these predictions would then be improved upon as machine learning approaches are developed and incorporated into the model. Thus, future technology for MIC diagnosis would have most of the necessary data built into the tool (model) so that the parameters that need to be obtained through sampling and analysis would be few, and the technology used to perform any analysis would be contained within one device. With accurate and reliable MIC diagnosis, prevention and mitigation measures could be more effectively applied, resulting in improved asset integrity, longevity, and sustainability.
This exclusive NACE International roundtable will continue with new commentary from additional panelists in part two of the series, available in December.
References
1 O. Gharbi, S. Thomas, C. Smith, N. Birbilis, “Chromate Replacement: What Does the Future Hold?” Materials Degradation 2, 1 (2018): p. 12.
2 https://www.questek.com/example-projects.html (accessed September 10, 2018).
3 Y. Qiu, S. Thomas, M.A, Gibson, H.L. Fraser, N. Birbilis, “Corrosion of High Entropy Alloys,” Materials Degradation 1, 1 (2017): p. 15.
4 M. Liu, Y. Guo, J. Wang, M. Yergin, “Corrosion Avoidance in Lightweight Materials for Automotive Applications,” Materials Degradation 2, 1 (2018): p. 24.
5 B. Hou, X. Li, X. Ma, C. Du, D. Zhang, M. Zheng, W. Xu, D. Lu, F. Ma, “The Cost of Corrosion in China,” Materials Degradation 1, 1 (2017): p. 4.