Nanotechnology is an industry that has recently begun to offer breakthrough solutions to the world. Accordingly, this article focuses on carbon nanotechnology advancements and their use in a new coating pioneered by Todd Hawkins, Jorma Virtanen, and the research and development team with Tesla NanoCoatings, Inc. (Massillon, Ohio). Their goal was to address the ever-growing demand from asset owners to obtain sustaining and economically viable coating solutions. The resulting coating is Teslan†, a two-part hybrid primer coating technology that chemically synthesizes carbon nanoparticles onto functional components of the formula to provide an assembled lattice of tough carbon nanowires within the resin.
The electrically conductive nature of the carbon nanowire lattice works in conjunction with either zinc, aluminum, or magnesium particles within the primers to combine superior physical performance properties of a barrier coating with a high degree of sacrificial cathodic protection (CP). Major time savings during the application process is also achievable due to unique rheological properties imparted by the nanoparticles, which allow two coats—the primer and epoxy topcoat—to be applied one immediately after the other in a wet-on-wet process.
Nanoscale carbon technology dates back to 1985 when Richard Smalley of Rice University (Houston, Texas) discovered the C60 Buckminsterfullerene molecule.1-2 Nanoscale carbon has the distinction of being the strongest material known to man, with a tensile strength 80 times greater than steel and a conductive capacity 1,000 times greater than copper. Carbon nanotubes and graphene are generally regarded as the stiffest and strongest material known, and these properties lend themselves to very useful applications in corrosion prevention and materials science. By harnessing the benefits of carbon nanotechnology, the developers were able to overcome the limited adhesion, flexibility, and impact resistance that can be associated with traditional metallic primer formulations, as well as improve coating system performance and life cycle costs.
During the curing process, the highly specialized allotropes of carbon used in the coating binder self-assemble into rope structures (nanowires) and form a reinforcing network throughout the coating. This network of carbon nanowires enhances the coating’s barrier properties by imparting the nanoparticles’ toughness and strength, which provide up to a 400% improvement in tensile and fatigue strength, and increases adhesion as well as impact and abrasion resistance. Years of testing and formulation optimization have enabled the researchers to create an optimized balance of resin to pigments. This has led to primers that yield highly impermeable barriers and resistance to microcracking, which prolong the coating system’s service life as a barrier coating.
In addition to their overall toughness, the carbon nanoparticles used in the galvanically active nanocoatings have a high electrical conductivity that is tuned, which enables the metallic primers to also function as a sacrificial coating in the event that the coating barrier is breached or degraded. The resulting electrically conductive quantum nanowire network is simultaneously in contact with the substrate and virtually all sacrificial metal particles.
Independent lab testing has indicated that only the zinc particles close to the substrate are actually active in providing CP in traditional zinc-rich coatings, despite the high concentration of metal.3 Nanoscale carbon solves this issue. The addition of the carbon nanoparticles facilitates a reduction of the amount of metal loaded into the primer. Because the carbon nanoparticles are conductive, the metallic particles will remain in electrical contact with each other and the steel substrate to provide sacrificial CP with a lower concentration of metallic dust (~50% less by volume as compared to a traditional zinc-rich epoxy).
The engineered use of carbon nanoparticles, with a very low percolation threshold (<0.1%), allows the amount of zinc particles to be reduced below their percolation threshold. This is possible because direct contact between zinc particles in the coating is no longer required for electrical conductivity when carbon nanoparticles are added. The carbon nanoparticle technology does not change the fundamental electrochemistry of the sacrificial CP, so the electrochemical reactions and redox potentials do not change. In other words, the carbon nanoparticles do not corrode at ambient temperatures; they remain inert and provide the quantum nanowire electrical network to better connect the substrate and sacrificial metal.
The service life of the sacrificial CP provided by the carbon nanoparticle technology in a zinc nanocoating vs. traditional zinc-rich epoxy was recently tested under an electrochemical test study.3 The results clearly demonstrated that use of nanocarbon extended the life of CP when compared to traditional zinc-rich epoxy.
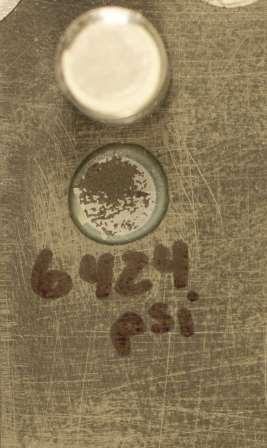 Since the use of carbon nanoparticles reduces the zinc pigment requirement, the functional barrier properties of the coating are also improved because pigment loading is well below the critical pigment volume concentration level. By reducing the metallic dust loading in the primer, the coating’s solids level is optimized and more resin is available to bond to the substrate. The resulting balance between the amount of pigment and the amount of resin in the coating improves the coating’s integrity and facilitates optimization of its barrier properties (such as surface adhesion, flexibility, abrasion and impact resistance, and tensile and fatigue strength), which are further enhanced with the reinforcing carbon nanowire network. Repeated adhesion testing of the zinc primer with carbon nanoparticles in accordance with ASTM D45414 consistently yielded values in excess of 4,000 psi (27,580 kPa) with the failure plane occurring in the Y layer (glue failure). The zinc/carbon nanocoating primer did not cohesively split or pull from the substrate (Figure 1).
Since the use of carbon nanoparticles reduces the zinc pigment requirement, the functional barrier properties of the coating are also improved because pigment loading is well below the critical pigment volume concentration level. By reducing the metallic dust loading in the primer, the coating’s solids level is optimized and more resin is available to bond to the substrate. The resulting balance between the amount of pigment and the amount of resin in the coating improves the coating’s integrity and facilitates optimization of its barrier properties (such as surface adhesion, flexibility, abrasion and impact resistance, and tensile and fatigue strength), which are further enhanced with the reinforcing carbon nanowire network. Repeated adhesion testing of the zinc primer with carbon nanoparticles in accordance with ASTM D45414 consistently yielded values in excess of 4,000 psi (27,580 kPa) with the failure plane occurring in the Y layer (glue failure). The zinc/carbon nanocoating primer did not cohesively split or pull from the substrate (Figure 1).
The porosity of the metallic primer is improved by the carbon nanoparticle network as well. Traditional zinc-rich coatings are porous and a large amount of each zinc particle is covered by an oxide layer that provides a continuous pathway for water and oxygen from the environment to move through the coating to the surface of the substrate, which works against the barrier property of the coating. The carbon nanoparticle technology resolves this issue. First, the particular type of carbon nanoparticle used in the coating inherently possess excellent barrier attributes that result in an overall improvement of the coating’s protective barrier properties. Additionally, when the amount of zinc is reduced, fewer pathways exist for moisture and oxygen to reach the steel, which improves the coating’s impermeability.
Much of the carbon nanoparticle primer development focused on solving the challenge of meeting the aging and immersion requirements for highly corrosive and harsh environments characterized by the high corrosivity categories in ISO 12944,5 specifically C5-M for environments such as inshore areas and offshore areas of high salinity (e.g., offshore marine structures). A two-coat system comprised of the carbon-fortified zinc primer and topcoat was tested according to the requirements of ISO 20340,6 and the results demonstrated that the coatings passed the aging requirements, with system performance exceeding that of a traditional three-coat zinc/epoxy/epoxy system. The carbon nanoparticle metallic primers are applied via traditional application equipment, and the primer and a compatible topcoat can be applied on the same day using Tesla NanoCoating’s 2x1 Wet Edge† system.
Source: Tesla NanoCoatings, teslanano.com. Contact Joe Davis, vice president of sales engineering, Tesla NanoCoatings, Inc.—e-mail: joe.davis@teslanano.com.
References
1 T. Hawkins, et al., “Smart Coatings and Nanotechnology Applications in Coatings,” ASM Handbook Volume 5B: Protective Organic Coatings (Materials Park, OH: ASM International, 2015), p. 193.
2 G. Gerlach, K.-J. Wolter, eds., Bio and Nano Packaging Techniques for Electron Devices (Berlin, Germany: Springer-Verlag Berlin Heidelberg, 2012).
3 V. Valencia-Goujon, et al., “Electrochemical Characterization in CO2 Saturated Environment of Zn-Rich Epoxy Nanocoatings on API X52 Pipeline Grade Steel Substrate under Flow Conditions,” CORROSION 2015, paper no. 6093 (Houston, TX: NACE International,
2015).
4 ASTM D4541-09e1, “Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers” (West Conshohocken, PA: ASTM, 2009).
5 ISO 12944-5:2007, “Paints and varnishes— Corrosion protection of steel structures by protective paint systems—Part 5: Protective paint systems” (Geneva, Switzerland: ISO, 2007).
6 ISO 20340:2009, “Paints and varnishes—Performance requirements for protective paint systems for offshore and related structures” (Geneva, Switzerland: ISO, 2009).
†Trade name.