Corrosion in reinforced concrete structures, such as bridges, piers, and parking garages, often occurs due to exposure to high-chloride environments. Those chlorides often come directly from the sea and impact the structure by immersion or a salt-laden atmosphere; or they might be brought to the structure in the form of deicing salts. One type of structure that is often overlooked is the manmade aquarium. Unlike bridge decks, which often experience the artificial ocean effects of deicers, aquariums quite literally have the ocean brought into them. Through specific case examples, this article discusses the unique challenges facing public aquarium owners and staff when addressing corrosion problems.
There are over 100 public aquariums in the United States. Like many public facilities, they have a number of operations obstacles to overcome, including public safety and security, water chemistry, and animal wellbeing. Also, like many public facilities, corrosion is not always a top concern until circumstances dictate otherwise. Furthermore, facilities and maintenance staff and aquarium designers and architects who are undereducated and misinformed about corrosion often exacerbate existing problems or create new ones. These professionals are often stretched thin through limited staff, budget shortfalls, and expectations to have at least a basic working knowledge of pumps and piping systems, water chemistry, plumbing, electricity, structural engineering, and, of course, corrosion. Too often the responsible individuals fit the old saying, "a jack-of-all-trades, master of none." Unfortunately, the complexity of corrosion issues at aquariums typically requires the attention of a master of that particular discipline. The following case examples describe common problems faced by aquarium owners and operators and methods that were used to mitigate those corrosion problems.
Case Example 1— Localized Rebar Corrosion
Large, reinforced concrete tanks are commonplace in public aquariums, and also are often susceptible to localized corrosion due to the environments they create. Construction joints in the concrete are functionally identical to small cracks, and wick in salt water from the tank interior to the reinforcement mat. This creates an environment with high chloride concentrations at the interior mat and low/no concentrations at the exterior mat. In addition, large percentages of tank exteriors are typically exposed to the air, either to provide visitor viewing locations or as part of the back-of-the-house areas. This creates an environment with high oxygen content at the exterior mat and low oxygen content at the submerged interior mat. These chemical concentration corrosion cells pose a challenging problem.
Equation (1) shows the typical cathodic reaction for steel in concrete:
(1) ½O2 + H2O + 2e ? 2OH
With high oxygen availability at the exterior rebar mat (visitor side) and low oxygen availability at the interior mat (water side), the interior mat will act anodically to the exterior mat because of limited cathodic reactants. Corrosion in the area of lower oxygen concentration might seem counterintuitive (everybody knows that exposure to oxygen causes steel to rust), but proper understanding of this phenomenon is fundamental to the study of corrosion. In addition, the presence of chlorides at the interior mat (especially at cracks and construction joints) will exacerbate that problem and create a very aggressive localized environment (Figure 1).
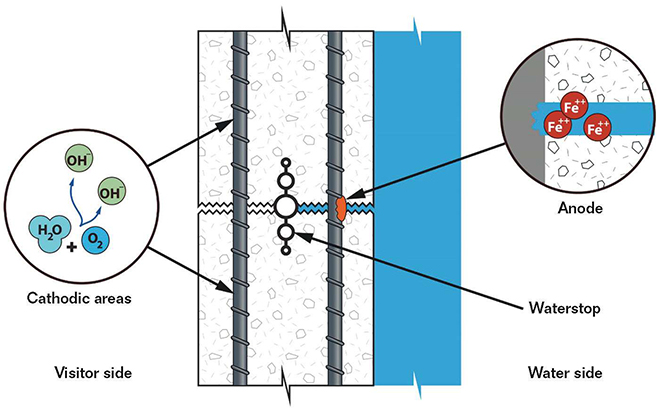
One of the major concerns about localized corrosion is an absence of visible cracking or spalling due to the buildup of corrosion product or red rust that could alert the observer to the presence of corrosion.
Relative to the bulk concrete, cracks can have a high average degree of wetness. If aggressive ions like chlorides are present, this wetness can facilitate movement of the chloride ions from the surface of the concrete to the surface of the rebar. Capillary suction can also play an important role.
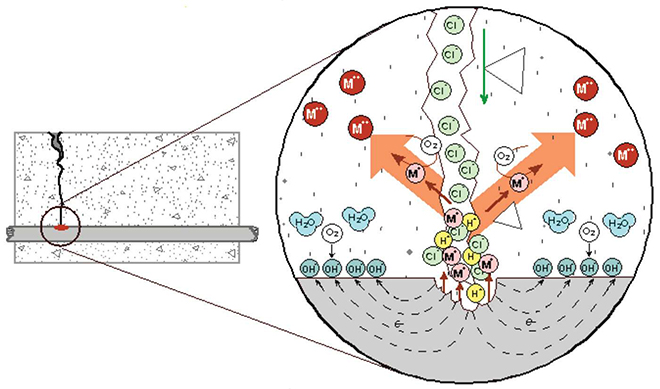
Figure 2 shows a schematic of the localized corrosion reaction that can occur at cracks in concrete where the external surface is exposed to chlorides. The key difference in localized rebar corrosion compared to general corrosion, which would produce spalls and delaminations, is that soluble corrosion products are able to be transported away from the steel surface, as shown in Equation (2):
(2) Fe0 ? Fe2 + 2e- (ferrous)
The iron, shown in Figure 2 as M+, goes into solution as Fe2+. As a soluble hydroxide, or as a chloride complex, the ferrous ion is able to move away from the surface of the parent steel. Completion of the oxidation process, as shown in Equation (3), and formation of voluminous red-rust corrosion products either occurs at the concrete surface (the crack opening) or within concrete pores or voids.
(3) Fe2+ ? Fe3+ + 1e- ( ferric)
For this specific case, corrosion potential mapping following ASTM C8761 was performed on a number of large reinforced concrete tanks holding the marine mammal exhibits for an aquarium in the Pacific Northwest. Even though the concrete showed no visible signs of corrosion and passed an acoustic sounding test, these tanks all had very negative corrosion potentials (-0.400 V or more negative vs. a copper/copper sulfate [Cu/CuSO4] electrode [CSE]) at the construction joint, but passive readings (-0.15 V or more positive vs. CSE) at all other locations. Even though the concrete "looked good," the potentials indicated localized corrosion, and rebar in these locations was exposed for visual inspection.
 Figure 3 shows what was left of the rebar after the exploratory exposure. At the construction joint, the rebar was completely consumed by localized corrosion, while the areas just above and below the construction joint still had full section. More rebar was exposed with similar results on every mammal tank. In fact, almost every rebar exposed showed >50% section loss.
Figure 3 shows what was left of the rebar after the exploratory exposure. At the construction joint, the rebar was completely consumed by localized corrosion, while the areas just above and below the construction joint still had full section. More rebar was exposed with similar results on every mammal tank. In fact, almost every rebar exposed showed >50% section loss.
To regain local building code compliance, new rebar was tied in and seismic braces were installed around the tanks. In addition, discreet galvanic anodes were installed along the length of the cold joint to prevent further localized section loss of the steel
Case Example 2—Corrosion Damage Caused by Cracking
Personnel at a small public aquarium in southern California had been trying to perform a quick fix on a leaking reinforced concrete tank for several years. Attempted repairs included caulking the leaking cracks and multiple attempts to cover them with cementitious material.
As in the previous case example, ASTM C876 corrosion potential mapping was used to find active corrosion on the tank wall around the cracking. Automated data collection equipment made it very quick work to obtain rebar corrosion potentials every 6 in (152 mm) on-center.
Figure 4 shows a potential map of two of the leaking cracks next to a photo of the structure. The corrosion potentials indicated elevated electrochemical activity at the cracks, especially on the rightmost crack in the photo.
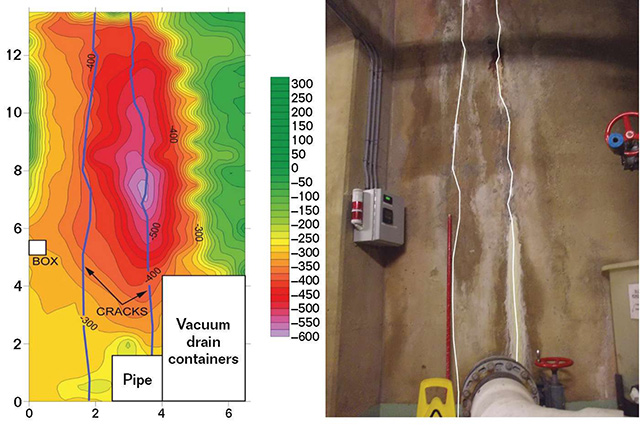
Exposing the rebar at select locations revealed that the majority of the rebar had >80% section remaining. However, a few locations had <50% section remaining. No corrosion damage was observed away from the cracking. As this is an area where earthquakes occur, locations with <75% remaining rebar section had new rebar tied in, and leaking and unsound concrete was replaced. Because corrosion was isolated to only a few cracked locations, a discreet cathodic protection (CP) system was also installed along the leaking cracks. Non-leaking cracks were injected with moisture-cure polyurethane foam.
Case Example 3—Stainless Steel Pitting and Crevice Corrosion
Austenitic stainless steels (SS), specifically Type 304 (UNS S30400) and 316 (UNS S31600), are sometimes used in salt water aquariums—typically as bolts, fasteners, or other hardware—due to the belief that they
are more corrosion resistant than mild carbon steels (CS). This is unfortunate because, while true in a variety of environments, it is not the case in chloride environments, particularly in low oxygen or anaerobic conditions. The ASM Metals Handbook indicates that “Type 304 and 316 SS suffer deep pitting if the seawater flow rate decreases below ~1.5 m/s (5 ft/s).”2 It further identifies crevice corrosion as the most problematic issue affecting the performance of steels in seawater. By their very nature of containing wildlife, surfaces in large seawater aquariums are prone to fouling, and that process creates its own crevice-like conditions.
Fontana and Greene define crevice corrosion as a type of corrosion attack associated with small volumes of stagnant water often found near holes, gaskets, lap joints, bolts, rivets, and even under deposits and other crevice-like areas.3 The stagnation and small mechanical gap prevent oxygen from entering the crevice. Oxygen will continue to be used as a reduction reactant within the crevice until it is consumed. Simultaneously, additional cations will be produced by the oxidation reaction within the crevice. Both of these events will create a net positive charge in the crevice electrolyte, which establishes a localized corrosion cell.
A public aquarium had recently constructed a new tank for large marine mammals. This tank was separated into several smaller tanks that could be joined or separated by the use of sliding gates. The gates were primarily windows with Type 316 SS gate frames, gate channels, and hardware. Above-water gate supports around the tank were also SS. Concern was raised when, after a short period of operation, significant corrosion product was observed around above- and below-water hardware, between the frames and windows, and between the channels and gates (Figure 5). This corrosion was consistent with crevice corrosion and localized only to crevice locations.

One of the problems with crevice corrosion is that it is difficult to mitigate. CP current does not easily flow into narrow crevices, and CP does not help at all on the above-water areas. Because this is an inhabited mammal tank, any potential coating work would be hindered, as each piece would need to be individually removed, coated away from the tank, and replaced.
In the end, it was agreed that the materials originally specified were simply the wrong materials. SS hardware was replaced with nonmetallic washers and titanium nuts and bolts. While the cost per titanium bolt was more than double the cost for SS, this was still an affordable option with fewer than 200 bolts to replace. SS framework and supports were replaced with nonmetallic alternatives where possible, and coated where replacement was not possible.
Conclusions
Public aquariums have a unique set of corrosion-related obstacles to overcome, which must be addressed to avoid early-onset corrosion problems and mitigate corrosion if it does occur. Material selection and maintenance strategies are critical in this endeavor; however, the importance of corrosion education for designers and facilities staff is also critical. Many of the
problems in these case examples could have been avoided or minimized if proper attention to corrosion had been given during design and installation.
References
- ASTM C 876, “Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete” (West Conshohocken, PA: ASTM International).
- “Properties and Selection: Irons, Steels, and High-Performance Alloys,” ASM Metals Handbook, Vol. 1, 10th ed. (Materials Park, OH: ASM International).
- M.G. Fontana, N.D. Greene, Corrosion Engineering, 2nd ed. (New York, NY: McGraw Hill, 1978), p. 39.
RYAN TINNEA is a project engineer with Tinnea & Associates, 2018 E. Union St., Seattle, WA 98122, e-mail: rtinnea@tinnea.net. He is a NACE International Cathodic Protection Specialist with 13 years of experience in corrosion consulting, including work on aquariums, piers, bridges, water and wastewater pipes and facilities, and dams. He is a 10-year member of NACE and vice chair of the NACE Publications Activities Committee.