Corrosion is a highly probable occurrence in water/wastewater facilities that can cause expensive repercussions and downtime. High levels of exposure to corrosive elements such as water, salt, acid, and chlorine are common, and there is a risk of irreparable damage to electrical equipment from corrosion. Therefore, electrical systems must be designed with the proper protection, and it is crucial to choose the proper products that will reliably perform and protect in such corrosive environments.
Conduit systems are designed to protect electrical wiring and equipment, although there are various options manufactured with different materials to choose from. Each type of conduit material produces a different reaction with environmental contaminants, and it is important to know which material will be the best at resisting highly corrosive areas within the water/wastewater environment.
Thus, there are two aspects to consider when choosing the right conduit product—the possible environmental contaminants the product will be exposed to, and how the conduit materials will behave when exposed to these elements. Understanding the reactions between the environmental elements and conduit material is crucial for preventing corrosion.
Improperly specified conduit and fittings will provide inadequate protection in a water/wastewater facility, causing electrical equipment to become subject to damage that can jeopardize control systems or possibly interrupt power to production equipment. When outages occur in facility equipment such as pumps, motors, lighting, or other components, maintenance and production costs go up, production losses go up, and profits go down. Therefore it is important to make informed decisions when designing electrical systems in all water/wastewater facilities. This article is intended to provide a foundation of knowledge that will aid end users, contractors, maintenance departments, and engineers in understanding effective corrosion prevention methods for highly corrosive environments.
Corrosive Elements
Knowing if a conduit product will provide reliable protection against corrosion begins with knowledge of the corrosive elements it will be exposed to. Below are some typical culprits of corrosion commonly found in water/wastewater facilities that require special attention when making specifications for conduit:
-
Sodium hypochlorite (NaClO) (bleach) is added to treated water to be used as “plant water.” NaClO pump stations are very corrosive, especially due to the delivery method that often results in spills. The product is typically delivered via tanker trucks, and the transfer upon delivery usually leads to spills and tank overflows. For this reason, there is always electrical conduit around the facility and pump stations. Protection of electrical wires is crucial, and polyvinyl chloride (PVC)-coated conduit and fittings are recommended to prevent early failure and high replacement costs caused by corrosion.
- Polymer is pumped into wastewater sludge as a thickening agent. A similar delivery procedure takes place with polymer, which usually results in spills and overflows. Again, PVC-coated conduit and fittings are a must in this type of area.
- Liquid oxygen is used in the industry to produce oxygen to feed, grow, and multiply microorganisms. An on-site liquid oxygen plant can always be seen due to the familiar fog rolling off of it. This means that the plant is in subzero temperatures that causes the electrical conduit to endure a lot of expanding and contracting, which can make wires vulnerable to the corrosive elements present within the facility. Using an Edison Testing Laboratories (ETL)-verified conduit helps maintain the structural integrity of a full conduit system.
-
Hydrogen sulfide (H2S) is obtained from waste-activated sludge, typically by a foul air system. H2S is highly corrosive and usually leaves a white crystalized residue on anything within its vicinity. The H2S will corrode galvanized metals and conduit. When the best performing PVC-coated conduit is specified and in place, contractors can usually wipe off this residue and see that the conduit is unharmed.
- Waste-activated sludge undergoes a process that turns it into thickened waste-activated sludge, which usually takes place in a domed structure. The interior of these domes is probably one of the most corrosive areas found in wastewater facilities. The H2S inside the dome is raw, which causes a dense air concentrate to form. Combined with the extremely high humidity inside the dome, moisture and H2S permeate the air to create a highly corrosive environment for electrical components. ETL-verified conduit is necessary to prevent product failure in such extreme conditions.
Conduit Materials
Conduit systems are usually manufactured with aluminum, rigid galvanized steel, stainless steel, PVC, or PVC-coated rigid galvanized steel pipe, each of which performs differently under various conditions. Some applications require a combination of these options to achieve proper protection. Therefore, an assessment and diagnosis of the environment must be made before choosing the appropriate conduit.
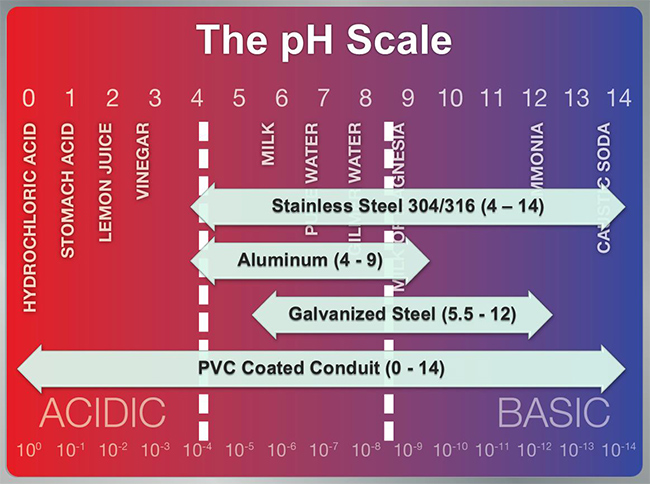
To assess the environment, pH is often used and measured on a scale of 0 to 14 (Figure 1). Low pH is acidic and high pH is basic or caustic. This is important, because measuring the pH in the environment leads to better material selection being made in the design process. Generally, if an operating environment is below 4 or above 8.5 on the pH scale, a more aggressive, corrosion-resistant material is needed. For conduit in particular, preventing corrosion in a wide application range requires the use of a PVC coating. Correctly applied coatings are going to seal the metal conduit from the corrosive environment. Utilizing a PVC coating on galvanized rigid conduit provides the corrosion resistance of PVC along with the strength of galvanized rigid conduit.
Protecting against Environmental Elements—The Basics
Even in mildly corrosive conditions, coatings are necessary to help conduit resist corrosion. Hot-dip galvanized steel conduit is a widely used, reliable choice for corrosive environments where wire and cable systems must be physically protected. It has been the standard in the industry for nearly 170 years and its performance abilities and limitations are well known. This is why a protective zinc coating covers most types of standard industry fittings more than any other material.
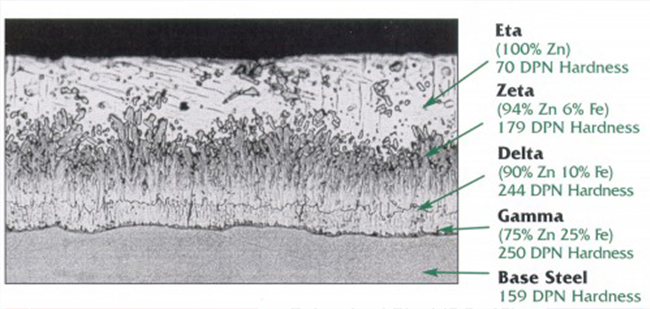
Hot-dip galvanized coatings are produced on conduit by immersing the cleaned conduit in a molten zinc bath. The steel and zinc react to form a metallurgically bonded coating that consists of a series of iron-zinc alloys, with increasing zinc content toward the external surface. Figure 2 shows a cross section of a galvanized coating and the various iron-zinc alloy layers. Zinc is naturally corrosion resistant in many environments, making it useful as a protective coating for a variety of applications. A fresh zinc surface is reactive to most environments and quickly forms a dense, adherent protective film—usually within the first 30 days of being exposed to corrosive elements. This film makes the corrosion rate of zinc 10 to 100 times slower than ferrous materials, which greatly increases corrosion protection.
However, this protection only lasts as long as the zinc itself, which erodes away over time as a result of exposure to various elements. Therefore, there is a standard for how thick a zinc coating must be in order to offer substantial protection in conduit systems. Rigid metal conduit standards such as UL 61 and ANSI C80.12 require that zinc coatings have a minimum thickness of 0.0008 in (0.02 mm).
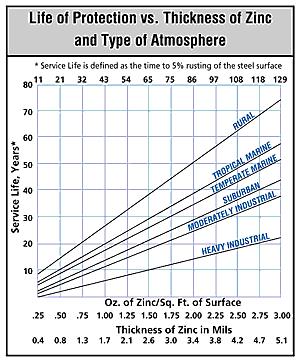 The graph in Figure 3 shows the average service life of a zinc coating at various thicknesses in a number of different environments. The service life is measured using the time the material was initially exposed to the respective environment until the time that 5% of the galvanized steel surface is rusted. The thickness of zinc is designated in mils on the graph, whereby 0.8 mils (20 µm) is equal to 0.0008 in, the standard minimum thickness of zinc for galvanized conduit. The data in Figure 3 are based on the historical performance of galvanized steel in each of the respective environments over the past century or more. The information on the graph has been widely used in the industry and is published in numerous technical articles and papers.
The graph in Figure 3 shows the average service life of a zinc coating at various thicknesses in a number of different environments. The service life is measured using the time the material was initially exposed to the respective environment until the time that 5% of the galvanized steel surface is rusted. The thickness of zinc is designated in mils on the graph, whereby 0.8 mils (20 µm) is equal to 0.0008 in, the standard minimum thickness of zinc for galvanized conduit. The data in Figure 3 are based on the historical performance of galvanized steel in each of the respective environments over the past century or more. The information on the graph has been widely used in the industry and is published in numerous technical articles and papers.
The data presented on the graph indicate that the location of the conduit has an undeniable impact on the rate of corrosion. In rural environments, the life cycle of a 0.8 mils-thick zinc coating is estimated to last 14 years; in temperate marine environments, it is reduced to eight years; in a moderately industrial environment, the coating will last about four years; and in a heavy industrial environment, the product is expected to last ~two years. In some particularly corrosive environments, the complete deterioration and disappearance of rigid steel conduit is reported in six months or less.
The zinc coating performs well under the extremely corrosive conditions of water/wastewater facilities, but additional options must be used to extend the performance life of the zinc.
Long-Term Corrosion Protection Options
Most of the time, water/wastewater facility owners and operators will not want to incur the high costs and downtime that comes with frequent replacement and installation of a new conduit system. Moreover, they will want to avoid the even more expensive replacement of damaged electrical equipment if the conduit fails to protect it from the harsh surroundings. Instead, it is much more reliable and cost efficient to choose materials that have undergone extra measures to increase the protective abilities of the product.
The best solution is the addition of a second coating—a different material layered over the top of the zinc to prevent the zinc surface from eroding.
PVC and Polyurethane Coatings
The best option for protecting galvanized steel conduit in severely corrosive conditions is an additional coating of paint or other polymer material, such as PVC. To protect the zinc and keep it from eroding, PVC is commonly added to galvanized conduit as a supplemental exterior coating and polyurethane (PUR) is applied to the interior to provide additional protection. PVC and PUR have excellent chemical resistance to acids and other chemicals that vigorously attack the galvanized zinc coating. Listed by Underwriters Laboratories (UL), such products have proven to provide excellent protection to the zinc in harsh industrial environments for several decades.
However, it is important to make sure the coatings are properly applied or their protective ability will fail and corrosive elements will reach the zinc. Therefore, proper manufacturing and adhesion of the coating to the zinc is crucial for reliable corrosion protection.
 Critical Surface Preparation for Adhesion
Critical Surface Preparation for Adhesion
In order for PVC and polyurethane coatings to properly adhere to a galvanized zinc surface, proper preparation of the surface is a must. Sherwin Williams states that approximately 80 to 90% of premature coating failures are caused by improper or inadequate surface cleaning and preparation.3 Most premature coating failures are caused by contaminants left on the surface when the coating is applied. Proper surface preparation of galvanized conduit for PVC and PUR adhesion is a very difficult task and it is important to make sure the manufacturing process is adequate. The Robroy Industries Conduit Division uses a two-step proprietary process—a mechanical surface treatment process to promote coating adhesion followed by a chemical cleaning process to remove any surface contaminants. All phases of both the mechanical and chemical processes are stringently controlled to provide surfaces that meet the highest standards for coating adhesion (Figure 4).
The Repercussions of Coating Failure
If the thickness of a PUR coating is inconsistent, or the adhesion of PVC fails, the zinc also becomes compromised. As seen in Figure 5, without proper coating adhesion, often times the corrosive elements become trapped underneath the coating and are held up against the substrate, which will accelerate the rate of corrosion. Additional exposure to moisture in a water/wastewater environment will cause these areas of white rust to continue to degrade, and eventually red rust will form when the underlying steel substrate begins to corrode.

Figure 6 shows a major adhesion failure of an external PVC coating. The adhesion of the PVC to galvanized zinc had failed in the bubble areas due to contaminants left on the surface before adhesion. The bond was broken and moisture penetrated the PVC, which caused the PVC to separate from the galvanized surface in a large area. The galvanized zinc was the only corrosion protection that remained for the rigid metal conduit.
 The zinc was then exposed to moisture and possibly other corrosive elements in the environment that had penetrated the coating. Once the separation occurred, corrosion would deteriorate the rigid steel conduit at a faster rate than uncoated galvanized conduit in the given environment because the moisture and contaminants became trapped.
The zinc was then exposed to moisture and possibly other corrosive elements in the environment that had penetrated the coating. Once the separation occurred, corrosion would deteriorate the rigid steel conduit at a faster rate than uncoated galvanized conduit in the given environment because the moisture and contaminants became trapped.
In such instances, nonvisible contaminants—usually water-soluble salts—are not completely removed from the zinc surface during the cleaning operation. When the coating is applied over salts remaining on the surface, the salts are hygroscopic and draw moisture through the coating. The moisture, in combination with the salts, creates a corrosion cell beneath the coating that begins to corrode the zinc, and the coating usually blisters, as shown in Figure 7.
 Summary
Summary
Reliable corrosion prevention is crucial for protecting electrical systems. For highly corrosive applications, the best performing conduit products consist of galvanized rigid steel, with interior and exterior coatings properly adhered to prevent exposure of the conduit.
Interior coatings must be applied with consistent thickness to prevent vulnerable thin areas where contaminates can penetrate and corrode the conduit. Likewise, PVC must be properly adhered to ensure that the zinc surface is protected from corrosive elements. For proper adhesion, stringent surface preparation such as the two-part proprietary system is very important, along with a rigorous quality assurance program.
The significance of coating adhesion has been recognized for years by corrosion engineers, but it was not documented in conduit performance standards until 2006. In 2006, an independent third party, Intertek ETL SEMKO, was engaged to develop a specification for a regulated and quantitative test protocol to confirm adhesion performance. The organization then independently performed a coated conduit evaluation based on the specification, and certified that Robroy Industries’ Plasti-Bond meets the requirements of the specification.
Tested and proven products have undergone proper manufacturing processes that make them a reliable choice for corrosion protection and long-term performance in the highly corrosive environment of water/wastewater facilities.
References
1 UL 6, “Electrical Rigid Metal Conduit—Steel” (Northbrook, IL: UL, 2007).
2 NEMA ANSI C80.1:2005, “Electrical rigid steel conduit (ersc)” (New York, NY: ANSI, 2005).
3 Sherwin-Williams, “Surface Preparation,” Resources, http://www.sherwin-williams.com/architects-specifiers-designers/products/resources/surface-preparation (September 25, 2015).
STEPHANIE ELLIS is the director of Corrosion College, 1100 U.S. Highway 271 S., Gilmer, TX 75644, e-mail: sellis@corrosioncollege.com.