In some applications, such as underground piping, temperatures within the range from 90 °C to 150 °C may be considered high-temperature. The focus of this article is significantly higher temperatures, primarily above 650 °C.
Ordinary iron-carbon alloys, including carbon steels, have been used successfully from room temperature up to temperatures of about 480 °C, and for short periods of time up to 590 °C. At temperatures beyond this, ordinary steels tend to corrode rather heavily, and the formation of surface oxide scales is ineffective at reducing these rates.
However, it is possible to resort to cementation (surface-diffusion treatments) on carbon and low-alloy steels to render them more resistant to corrosion. Depending upon the corrosiveness of the medium involved and the exposure temperature, one or several of the following may be used: siliconizing, aluminizing, chromizing, or a somewhat different method of surface treatment such as plating, plasma spraying, cladding, and so forth.
The most common alloys used for exposure at high temperatures are the iron/nickel/chromium alloys, often referred to as stainless steels (SS). The materials used may be cast or wrought. For most furnace operations, the cast metals are used.
If medium- or high-alloy steels are used, chromium-bearing SS are generally recommended. Both the straight chromium steels (400 series) and the chromium/nickel/steels (300 series) are used successfully up to approximately 870 °C.
At still higher temperatures, the higher nickel and higher chromium alloys of the 300 and 400 series are used, and at temperatures in excess of 1,100 °C, only the alloys with more than 20% Cr can be used with relative safety, particularly for short or intermediate periods of time.
During long-term use at high temperatures, a phenomenon known as creep is experienced, during which relatively low stress may cause a metal to deform very slowly and possibly rupture. The percent elongation of a metal component is called strain, and the strain per unit time—typically not a constant at high temperatures—is called creep.
Although this is not a corrosion phenomenon, it is nevertheless a factor that must be understood by anyone considering or recommending alloys for use at very high temperatures. It is on the basis of the creep to be expected at the operating temperature that furnace parts are designed to withstand the mechanical stress.
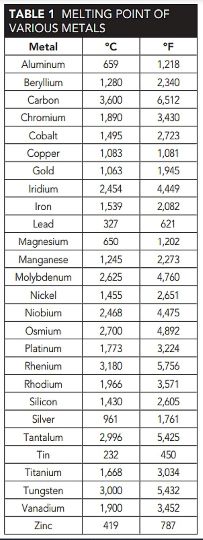
At still higher temperatures, it may be necessary to use refractory metals that have good high-temperature mechanical properties, plus melting temperatures greater than 1,650 °C, as shown in Table 1. Alloys of chromium, niobium, molybdenum, tantalum, rhenium, or tungsten will therefore be considered.
Although titanium and zirconium melt above 1,650 °C, their high-temperature mechanical properties are rather unsatisfactory, so they are not generally considered in the category of refractory metals. Although pure vanadium melts near 1,900 °C, the low melting point of its oxide makes it a poor choice for high-temperature applications.
While alloying with metals that melt at higher temperatures will generally produce a higher-melting material, the resulting properties and high-temperature performance of specific candidate alloys must be fully understood.
This article is adapted from Corrosion Basics—An Introduction, Second Edition, Pierre R. Roberge, ed. (Houston, TX: NACE International, 2006), pp. 218–219.