Modern automotive coatings do more than just sparkle and shine.
Along with aesthetic appeal, they provide long-lasting protection against corrosion.
Now researchers at Texas A&M University in College Station, Texas, USA—sponsored by Toyota Motor North America— have developed a groundbreaking approach to predict the long-term performance of automotive surface coatings.
“Beyond adorning cars with an attractive finish, these coatings act as a protective shield against the hazards encountered on the road. They shield the metal body from corrosion, absorb the impact of stone chips, resist scratches, and provide a barrier against the harsh effects of chemicals and pollutants, thereby prolonging the life and allure of the vehicle,” the researchers write in their AMPP conference paper “A Damage-Based Approach and Predictive Modeling of Corrosion in Substrate/Coating Systems Using Artificial Neural Networks.”
Their research introduces the use of artificial neural networks (ANN)—which mimic the human brain’s ability to learn—combined with electrochemical impedance spectroscopy (EIS) and validated by scanning electron microscopy (SEM). These methods help to forecast corrosion in automotive coatings, which could lead to significant advancements in vehicle durability.
“You want to have a very beautiful car, shiny and scratch-less,” says Homero Castaneda-Lopez, director of Texas A&M’s National Corrosion and Materials Reliability Laboratory. “But you also want to have a good operation, a reliable car. If you can know which materials are more durable, you’re going to have more trust in the cars, not only how beautiful they look, but how trustworthy they are, especially in difficult weather conditions.”
Their research focuses on three types of automotive coatings: physical barrier coatings, sacrificial metallic coatings, and hybrid coatings that combine elements of both. These coatings are essential in protecting vehicles from the harsh effects of environmental exposure, such as deicing salts, which can lead to corrosion and material degradation over time.
“You always try to simulate or mimic the conditions in the field,” Castaneda says. “You will not have the same conditions, but you will have trends and patterns that are used to understand the real environment. The lab gives you leads, but it’s important that you follow what’s happening in real conditions. This project touches that.”
The paper’s authors presented their findings at the AMPP Annual Conference + Expo in March in New Orleans, Louisiana, USA.
In addition to Castaneda and Heather Eich, senior engineer at Toyota North America, Victor Ponce and Shaik Merkatur Hakim Marjuban of Texas A&M and Walter Tarr and Allison Mahood of Toyota contributed to the paper.
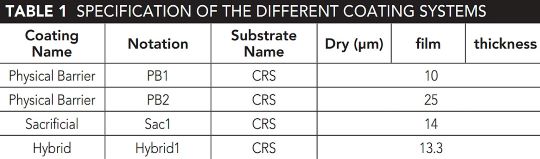
Three Types of Coatings
The researchers studied three different types of coatings—physical barrier coating, sacrificial coating, and hybrid coating.
“We are testing to see which of these three concepts are more suitable for the automotive industry by considering where these systems are going to be exposed,” Castaneda says.
They immersed samples of these coatings in a salt solution to simulate real-world conditions. They then used EIS to monitor how the coatings degraded over time, tracking changes in their protective abilities. This data was used to train an ANN model, a type of artificial intelligence (AI) that predicted how these coatings would perform in the future.
“We believe that the tool we developed is going to be able to distinguish or screen each coating within a short period of time, let’s say 30 or 40 days, and we will be able to see if that coating is going to last many years,” Castaneda says.
Their lab findings are highly accelerated, reducing what once took four to six months to as few as 35 days.
Eich explains that there is no exact correlation between simulated lab conditions and years of real-world corrosion protection.
“People always want that magic answer,” she says. “How many days in the field is this? But it’s really difficult to get to that exact amount.”
The Ohio State University graduate in mechanical engineering explains that Toyota conducts parallel field studies to verify the lab findings.
“We have 10 field monitor vehicles that we launched in 2022 with duplicate test panels to what we’re running in the lab,” she says. “We’re running them in high-salt-usage regions throughout the U.S. and Canada. They have test panels underneath their vehicles that they’re driving every day, and we’re running the same evaluations that we are in the lab in the field.”
Physical Barrier Coating
The physical barrier coating is a protective layer that acts as a barrier to prevent water and other harmful substances from reaching the metal underneath. Greater thickness provides greater protection, but at a cost.
“The barrier coating is a material that prevents the interaction from the environment with the metal we call the substrate,” says Castaneda, who has a Ph.D. in materials science and engineering from Penn State University. “That physical barrier not only prevents the interaction, but also the potential damage because of that interaction.”
The researchers observed that as time passed, water slowly penetrated the coating. Initially, the coating did a good job of keeping water out, but eventually, the water reached the metal underneath. The thicker the coating, the better it performed. The ANN model (Figure 1, top) predicted that after about 120 days of lab time, the water would start to react with the metal, forming a protective oxide layer that would stabilize the system.
Sacrificial Coating
A sacrificial coating is made from a metal that corrodes before the underlying metal does, thus protecting it.
“Sacrificial means that you would rather damage or deteriorate that coating instead of the substrate, so you put another coating that protects by reacting with that coating, so it sacrifices itself to prevent the interaction from the environment to the substrate.”
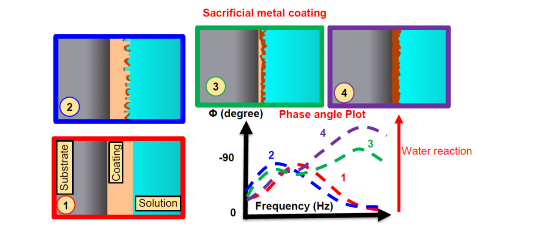
When exposed to the salt solution, the sacrificial metal in the coating started to corrode first, forming a protective layer over the underlying metal. This process happened over about 80 days of simulated real-world conditions in the lab, after which the sacrificial layer was mostly used up, leaving behind a stable protective oxide layer. Their model (Figure 2) successfully predicted this progression.
Hybrid Coating
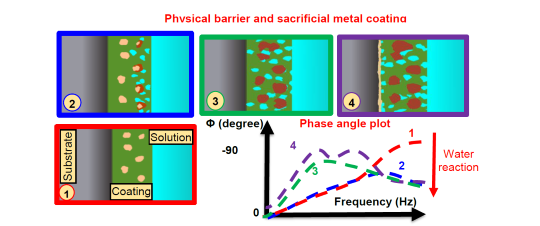
The hybrid coating is a combination of the first two types, offering both a physical barrier and sacrificial protection.
“Here we have a material that is not that thick, but is thick enough to have a polymer base material, but also you have another material embedded in the polymer that is sacrificial,” Castaneda says.
It combines the benefits of both physical barrier and sacrificial coatings and shows the most complex behavior. Initially, it acted like a physical barrier, but as time went on, the sacrificial metal within the coating started to corrode, providing an extra layer of protection. The team’s predictions (Figure 3) indicated that this coating could offer longer-lasting protection than either of the other types alone.
SEM Validation
The research team used SEM analysis to provide visual confirmation of protective mechanisms of the coating systems. Figures 4 through 7 show results from physical barrier 1 at 10 µm, physical barrier 2 at 25 µm, the sacrificial barrier at 14 µm, and the hybrid barrier at 13.3 µm.
“SEM images captured on day 1 revealed the absence of any discernible interphase layer between the coating and the substrate,” the authors write about the 10 µm barrier (Figure 4). “However, an intriguing transformation was observed on day 120, where the unmistakable appearance of a passive layer became evident.”
Images of physical barrier 2 (Figure 5) “do not reveal the presence of a passive layer between the substrate and the physical barrier coating. This observation suggests that the enhanced thickness of PB2 provides effective protection, in contrast to PB1.”
The sacrificial barrier’s SEM image (Figure 6) taken on day 1 does not indicate any signs of a reaction within the metallic layer or a passive layer on the substrate surface. “However, a significant transformation becomes evident by day 60, where the sacrificial metallic layer exhibits full reactivity.”
The hybrid barrier day 1 image (Figure 7) provides no indications of a reaction involving the sacrificial metallic particles embedded within the coating layer. “However, a notable transformation becomes evident by day 30, where the sacrificial metallic particles exhibit full reactivity. … The SEM image taken on day 80 reveals the corrosion of the substrate, which subsequently leads to the formation of a passive layer.”
Future Applications
Although these corrosion prevention techniques may be invisible to consumers, they expect the best protection, Eich says.
She says this research not only will benefit auto manufacturers and customers in the future but will translate to other industries such as marine and aerospace. For now, her focus is on her organization’s customers.
“It may be something that our customers don’t see—you’re not often looking underneath your vehicle—but you are counting on those parts to perform,” she says. “We want to make sure that we’re selecting the right coatings and the right applications and continuing to get the best technology out there so we can exceed our customers’ expectations for corrosion.”
Editor’s note: This article first appeared in the October 2024 print issue of Materials Performance (MP) Magazine. Reprinted with permission.