Editor's note: Learn more about coating aluminum
substrates in this new
Materials
Performance special feature, “Science in Action.” After you've read the MP article about the science of forming
corrosion-resistant MAO coatings on an aluminum alloy, explore the application
of protective coatings on aluminum, which is presented in several related
CoatingsPro Magazine features listed at
the end of the article.
Micro-arc oxidation (MAO), a relatively new surface modification technique for light alloys such as aluminum, magnesium, titanium, and their alloys,1-3 has attracted considerable attention in recent years. The MAO treatment produces a ceramic coating on aluminum alloys that enhances their wear resistance4 and anticorrosion properties.1
The MAO process is carried out at voltages higher than the breakdown voltage of the gas layer enshrouding the anode. Since the substrate alloy is connected to the positive pole of the rectifier as the anode, the gas layer consists of oxygen. The coating formed on the substrate alloy is comprised of crystalline or amorphous phases, formed at breakdown sites, and usually contains constituent species derived from the substrate and the applied electrolyte. Research work shows that the properties of MAO coatings depend on electrical parameters,4-5 type of power source,6 oxidation time,7 and additives.8-9
Aluminum alloys have received much attention in the machinery and transportation industries, especially for aerospace and automobile products, due to their advantages of high strength-to-weight ratio and light weight.10 However, their poor anticorrosion properties in some environments have greatly limited their applications. Therefore, it is very important to improve their surface properties and corrosion resistance by surface treatment modifications.
In this study, a MAO treatment was performed on ZL101A (UNS Al3560) aluminum alloy in a silicate-based electrolyte with and without the addition of aluminum oxide (Al2O3). Potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) were conducted in a 3.5 wt% sodium chloride (NaCl) solution to study the corrosion resistance of the MAO coatings formed on the ZL101A aluminum alloy.
Experimental Procedure
ZL101A aluminum alloy was used as the substrate material in this study, with the following composition: 6.5 to 7.5 wt% Si, 0.19 wt% Fe, 0.05 wt% Cu, 0.1 wt% Mn, 0.45 to 0.7 wt% Mg, 0.07 wt% Zn, and the remainder Al. Cylinder specimens (3-mm thick and 40 mm in diameter) were used as the substrate. The surfaces of the specimens were successively ground with 200, 500, 800, and 1,200 grit waterproof alumina abrasive paper and then ultrasonically cleaned in ethanol for oil removal. Prior to the MAO treatment, the specimens were cleaned in distilled water and dried in hot air.
The MAO treatment was carried out using a pulsed direct current power source. The specimens were installed as the anode and a stainless steel barrel served as the cathode. A sodium silicate (Na2SiO3)-based aqueous solution—Na2SiO3 (18 g/L) + potassium hydroxide (KOH) (3 g/L) + sodium fluoride (NaF) (4 g/L)—was used as the base electrolyte, and the concentration of Al2O3 particles (<20 nm) in the electrolyte solution varied from 3 to 9 g/L with +2 g/L intervals. MAO coatings were produced by a pulsed current with a set current density of 150 mA/cm2 for a 40-min time interval.
The elemental composition of coatings with and without 7 g/L additive Al2O3 were examined by x-ray photoelectron spectroscopy (XPS) with an Al Kα radiation (λ=1486.6 eV). The x-ray photoelectron spectroscopy XPS analysis was conducted after the film surfaces were etched for 1 min by an argon-ion beam to reduce carbon contamination. All energy values were corrected according to the adventitious C 1s signal, which was set at 284.6 eV. The data were analyzed with Xpspeak 4.1† software.
The electrochemical tests were carried out in 3.5 wt% NaCl, with a three-electrode system. The uncoated and MAO-coated specimens were used as the working electrodes, and a weight-saving platinum electrode and a saturated calomel electrode (SCE) were used as the counter electrode and reference electrode, respectively. The working electrode (100 mm2 area) was exposed to 3.5 wt% NaCl for 30 min to stabilize its corrosion potential (Ecorr). The potentiodynamic polarization test was carried out from -1.2 V to -0.2 V vs. a SCE at a scanning rate of 1 mV/s. An EIS measurement was carried out on the specimen at its corrosion potential. A 10-mV peak-to-peak amplitude potential signal was selected. Frequency range was from 105 to 10-1 Hz.
Results and Discussion
XPS Results
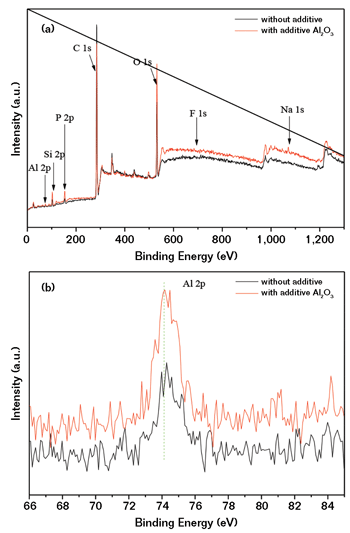 The effect of the Al2O3 additive on the MAO coatings formed on the ZL101A aluminum alloy was estimated by the chemical state of the coatings’ surface. The full range and Al 2p core level XPS spectra of the MAO coatings formed in the additive-free and 7g/L additive-containing electrolytes are shown in Figures 1(a) and (b), respectively. As shown in Figure 1(a), both coatings are composed of C, Al, Si, P, O, F, Na with photoelectron peaks appearing at binding energies of 284.82 eV (C 1s), 74.36 eV (Al 2p), 102.73 eV (Si 2p), 133.3 (P 2p), 531.92 eV (O 1s), 685.4 eV (F 1s), and 1,071.84 eV (Na 1s), respectively. The appearance of a carbon peak is attributed to the residual carbon from the precursor solution or the surface adventitious carbon.11
The effect of the Al2O3 additive on the MAO coatings formed on the ZL101A aluminum alloy was estimated by the chemical state of the coatings’ surface. The full range and Al 2p core level XPS spectra of the MAO coatings formed in the additive-free and 7g/L additive-containing electrolytes are shown in Figures 1(a) and (b), respectively. As shown in Figure 1(a), both coatings are composed of C, Al, Si, P, O, F, Na with photoelectron peaks appearing at binding energies of 284.82 eV (C 1s), 74.36 eV (Al 2p), 102.73 eV (Si 2p), 133.3 (P 2p), 531.92 eV (O 1s), 685.4 eV (F 1s), and 1,071.84 eV (Na 1s), respectively. The appearance of a carbon peak is attributed to the residual carbon from the precursor solution or the surface adventitious carbon.11
The Al 2p core level spectra, as shown in Figure 1(b), illustrate that Al 2p spin-orbit components of the 7g/L additive-containing coatings are shifted toward higher binding energy, as compared to the additive-free coating. This suggests that some of the Al2O3 additive is incorporated into the coating.
Electrochemical Tests
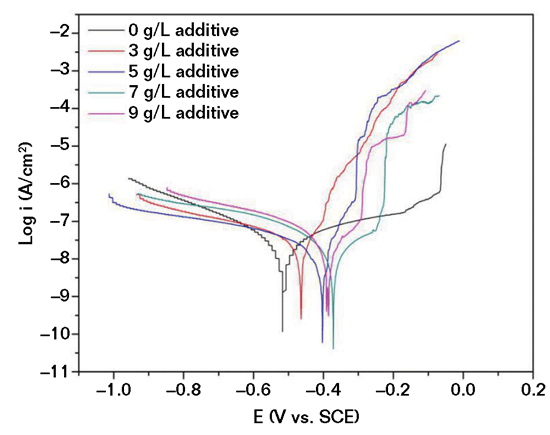
Potentiodynamic polarization curves of the uncoated and MAO-coated aluminum alloy are shown in Figure 2. As the concentration of Al2O3 increased in the electrolyte, the potentiodynamic polarization curves shifted to the right (a more noble corrosion potential) and then turned to the left (a lower corrosion potential). The MAO coating formed in the 7g/L Al2O3-containing electrolyte showed the highest corrosion potential of -0.375 V and a corrosion current density of 1.819×10-9 A/cm2. However, when the Al2O3 additive concentration increased to 9 g/L, the corrosion potential of the coating decreased and the corrosion current density increased. This indicated that the coating formed in the 7g/L Al2O3-containing electrolyte provided the most anticorrosion properties.
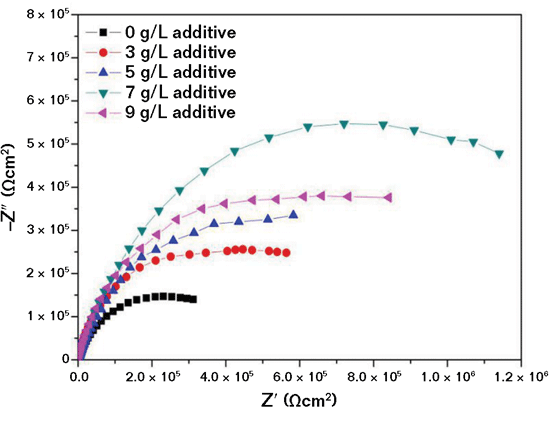
The corrosion behavior of the uncoated and MAO-coated aluminum alloys in 3.5 wt% NaCl aqueous solution was further studied by EIS tests. The Nyquist plots are shown in Figure 3. A similar evolution process was observed, which indicates that a similar corrosion mechanism was in effect. The corrosion rates were evidently different due to the greater radius of the capacitive loops (i.e., a larger-radius capacitive loop corresponds to a lower corrosion rate). The MAO coating obtained in the 7 g/L Al
2O
3-containing electrolyte exhibits the largest capacitive loop of all the MAO coatings, indicating its superior ability to protect the aluminum alloy from corrosion vs. that of the MAO coating obtained in
Al2O3-free electrolyte. When the Al2O3 additive concentration increased to 9 g/L, the radius of the capacitive loop decreased. This indicates that the corrosion resistance of the MAO coating formed in the 9 g/l Al
2O
3-containing electrolyte is inferior to the coating formed in the 7 g/l Al
2O
3-containing electrolyte. This is essentially in agreement with the relationship between the potentiodynamic polarization test and EIS results.
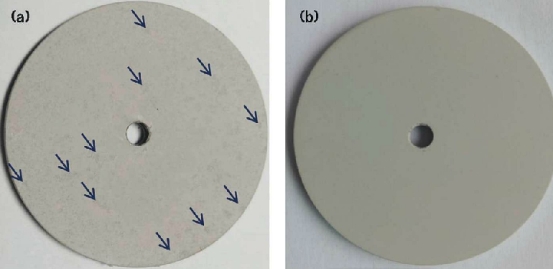
Long-Time Immersion Test
Photographs taken of the MAO coatings formed in the Al2O3-free electrolyte and 7 g/L additive-containing electrolyte after a salt immersion test in a 5 wt% NaCl aqueous solution for 96 h are shown in Figure 4. The coating formed in the electrolyte without the Al2O3 additive presented some scattered black areas, as seen in Figure 4(a). In contrast, the MAO coatings formed in 7 g/L additive-containing electrolyte show no obvious black areas on the surface, as seen in Figure 4(b). This proves that the anticorrosion properties of the MAO coatings formed in the 7 g/L additive-containing electrolyte are much better than those of the coating formed in the additive-free electrolyte after 96 h immersion in 5 wt% NaCl solution. This observation is in good agreement with the electrochemical analysis results.
Conclusions
MAO coatings formed on ZL101A aluminum alloy with the addition of various concentrations of Al2O3 were investigated. The elemental composition and corrosion resistance of MAO-treated specimens were studied. The following conclusions can be drawn:
1. XPS results revealed that Al 2p spin-orbit components of the additive-containing coatings shifted toward higher binding energy, which suggests that some of the Al2O3 additive is incorporated into the coating with respect to the additive-free coating.
2. Electrochemical tests indicated that Al2O3 is an efficient additive with the ability to improve the corrosion resistance of MAO coatings formed in an alkaline, silicate-based electrolyte. The coating formed in the 7 g/L Al2O3-containing electrolyte exhibited the best anticorrosion properties.
3. Long-time immersion tests showed that the MAO coating formed in the 7 g/L Al2O3-containing electrolyte showed no obvious black areas. However, the coating formed in the Al2O3-free electrolyte presented some scattered black areas after the immersion time of 96 h.
Acknowledgments
The financial aid of Research on Public Welfare Technology Application Projects of Zhejiang Province under grant no. 2017C31039 and the National Natural Science Foundation of China under grants no. 51275477 and no. 61572438 are gratefully acknowledged.
†Trade name.
References
1 H Fadaee, M. Javidi, J. Alloys Comp. 604 (2014): pp. 36-42.
2 P.B. Srinivasan, et al., Appl. Surf. Sci. 256 (2010): pp. 3,265-3,273.
3 Z.P. Yao, et al., Surf. Coat. Technol. 269 (2015): pp. 273-278.
4 N. Xiang, et al., Trans Nonferrous Met. Soc. China 26 (2016): pp. 806-813.
5 K.R. Shina, et al., Appl. Surf. Sci. 314 (2014): pp. 221-227.
6 K.Q. Du, et al., Mater. Lett. 91 (2013): pp. 45-49.
7 M. Montazeri, et al., Appl. Surf. Sci. 257 (2011): pp. 7,268-7,275.
8 T.S. Lim, H.S. Ryu, S.H. Hong, Corros. Sci. 62 (2012): pp. 104-111.
9 J. Liang, et al., Appl. Surf. Sci. 252 (2005): pp. 345-351.
10 Z.J. Wang, et al., Surf. Coat. Technol. 204 (2010): pp. 3,315-3,318.
11 C. Quinones, W. Vallejo, J. Ayala, Appl. Surf. Sci. 257 (2010): pp. 367-371.