Graphene, due to its exceptional electrical and mechanical properties, has attracted attention as a potential material for imparting anticorrosive performance. Graphene is a carbon allotrope with a two-dimensional molecular structure comprised of a single layer of carbon atoms bonded together in a hexagonal lattice structure. Bohm1 proposed that graphene’s two dimensional platelet structure enables excellent performance in barrier coatings based on its high surface area, electrical conductivity, and impermeable nature.
This enhanced performance may be explained by the combination of three characteristics: graphene makes the path of water permeation more difficult; the impermeability of graphene’s molecular structure reduces the penetration of oxygen, water, and other corrosive materials; and graphene provides an alternative path for electrons and breaks the electrochemical cell that is necessary for corrosion. Subsequently, graphene has been investigated to explore its corrosion-resistant capabilities.
Work has included doping graphene with corrosion inhibitors1 and depositing it on substrates using chemical vapor deposition (CVD). Potentiometric analysis and traditional corrosion testing has suggested that graphene can provide significant performance improvement.2, 3 A large part of this work has been done with discrete layers of graphene grown via CVD or applied directly to surfaces.
Applied Graphene Materials (AGM) (Cleveland, United Kingdom) has developed and patented a unique graphene synthesis process to produce dispersion-ready graphene nanoplatelets using sustainable raw material sources rather than exfoliating graphene from graphite. Development work is underway that explores the ability of graphene nanoplatelets distributed throughout a coating film to function in a comparable manner to a monolayer of graphene applied by CVD, and evaluates their ability to prevent corrosion. Such an approach could open up the opportunity for coating manufacturers to utilize graphene when formulating coatings with improved performance.
Independent industry experts Paint Research Association (PRA) (Leicestershire, United Kingdom) and The Welding Institute (TWI) (Cambridge, United Kingdom) worked with AGM to complete an evaluation of graphene nanoplatelets in an epoxy coating, with the aim of demonstrating how a graphene-enhanced coating may be useful in preventing corrosion.
Two grades of graphene platelets were evaluated—a medium density graphene with a rigid platelet structure and built-in oxygen functionality that provides good dispersibility (Sample 1), and an ultra-low-density, high-surface-area graphene that has a flexible, crumpled sheet morphology (Sample 2). These materials were selected because they each have properties that can be useful in preventing corrosion. A two-pack epoxy system, cured with a proprietary unmodified aliphatic amine hardener system, was selected to represent epoxy primer systems used to protect steel and aluminum structures.
The graphene was dispersed directly into the resin at loading levels that ranged from 0.1 wt% to 5.0 wt%. Sample 2 was limited to a maximum loading level of 1.0 wt% due to its very-high surface area. The graphene coatings were applied to mild steel panels that were hand-abraded according to ISO 15144 and cleaned with xylene. The panels were then cured for seven days at 18 to 25 °C to create a dry film thickness of 40 µm for testing using a draw-down method.
Cyclic corrosion resistance was tested according to BS EN ISO 11997-25 modified to remove ultraviolet light exposure. Using a repeating cycle for a total of 1,000 h, duplicate specimens were exposed for 60 min to dilute electrolyte fog (0.35% ammonium sulfate [(NH4)2SO4] and 0.05% sodium chloride [NaCl]) at ~24 °C followed by 60 min drying time with temperatures rising to 35 °C. Panels were checked regularly to monitor the progression of corrosion and were rated at three and six weeks for defects such as blistering and rusting following the guidelines of ISO 4628-2.6
Figure 1 shows representative images of a selection of panels before and after 1,000 h of salt fog testing. The graphene-loaded epoxy-coated samples remained corrosion free for up to 12 days, and then only showed very small, localized spotting. This localized corrosion appeared to be limited to regions where there had been defects or pitting in the coating. There was also an increase in the time it took for the onset of corrosion—the appearance of black rust spots—with increased graphene loading. The best performance was achieved with 5.0% of Sample 1 and 0.5% of Sample 2.
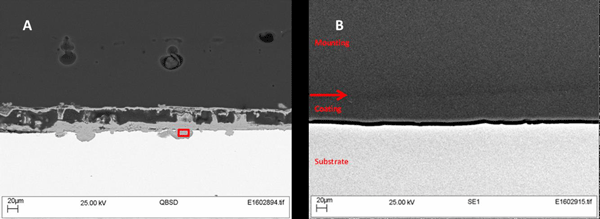
Following the positive results in the cyclic salt fog testing, the graphene-enhanced epoxy coatings were subjected to immersion testing. Steel panels, hand-abraded and cleaned with xylene, were fully immersed in synthetic seawater (prepared according to ASTM D11417) at ambient temperature (20 to 30 °C) for 30 days. Upon completion of the immersion testing, samples were cross-sectioned and imaged by scanning electron microscopy (SEM).
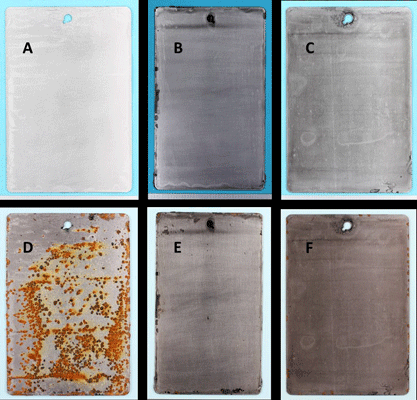
Figure 2 shows photographs of the epoxy-coated steel panels before and after the 30-day immersion in synthetic seawater. The panel with the graphene-free control epoxy suffered severe corrosion and rusting, while the panels with the graphene-loaded epoxy were virtually corrosion free. The graphene significantly enhanced the corrosion mitigation of the epoxy coating even at loading levels as low as 0.1 wt%. Typically, corrosion mitigation improved as the loading level of graphene increased. SEM images for Sample 2 (Figure 3) show the coating remained intact, with no rusting or corrosion visible at the surface of the substrate. The diffusion of water and salts to the surface was prevented by the graphene in the coating.
Because corrosion is an electrochemical process—an electrical current is produced as the metal in the substrate is oxidized—electrochemical monitoring of a substrate during immersion testing provides useful information about how well the coating is protecting the steel panel. Monitoring this electrical current can quantify the amount and rate of corrosion. In experiments with electrochemical testing, panels coated with graphene-loaded epoxy were immersed in synthetic seawater and measurements taken using a three-electrode system. The corrosion current for each sample was monitored over the 30 days of immersion.
The corrosion current recorded for the samples coated with graphene-loaded epoxy was roughly 1,000 times smaller than for the control sample coated with graphene-free epoxy. This very low corrosion current correlates with the conclusions from the visual assessment and the SEM analysis, and confirms that the addition of graphene to the epoxy improves the corrosion protection provided by the epoxy coating.
The proposed explanation for this is that the graphene nanoplatelets act as a barrier to the diffusion of water and corrosive salts through the epoxy coating. The water vapor transmission rate (WVTR) through the epoxy coatings was measured following ASTM D1653-038 using Test Method B (wet cup method), Condition A (23 °C, 50% relative humidity). Samples of graphene-free epoxy and epoxy loaded with Sample Grade A and Sample Grade B were coated onto a paper substrate for this test.
The results of the WVTR test showed a clear reduction in the diffusion rate of moisture through the graphene-loaded epoxy samples. The data show that the graphene-free control epoxy had a WVTR of ~200 g/m2/day. The addition of the smallest amounts of graphene tested, 0.1 wt%, reduced this diffusion rate by a factor of almost 100. A WVTR of less than 10 g/m2/day was recorded for all graphene-loaded epoxy samples.
The data support the proposed mechanism that the graphene nanoplatelets form a very effective diffusion barrier by effectively forming a tortuous path to the substrate and greatly increasing the time it would take for corrosive elements to migrate through the coating. The effective barrier properties of the graphene nanoplatelets in the epoxy coatings may explain the corrosion mitigation observed on the steel panels in both immersion testing and cyclic salt fog testing.
Source: Lynn Chikosha, Adrian Potts, and William Weaver, Applied Graphene Materials, appliedgraphenematerials.com. Contact Marie Robinson—email: marie.robinson@appliedgraphenematerials.com.
References
1 S. Bohm, “Graphene against Corrosion,” Nat. Nanotechnology 9, 10 (2014): pp. 741-742.
2 R.S Raman, et al., “Protecting copper from electrochemical degradation by graphene coatings,” Carbon 50, 11 (2012): pp. 4040-4045.
3 R.V. Dennis et al., “Graphene nano-composite coatings for protecting low alloy steels from corrosion,” Am. Ceram. Soc. Bull. 92, (2013): pp. 18-24.
4 ISO 1514:2016, “Paints and varnishes—Standard panels for testing” (Geneva, Switzerland: ISO, 2016).
5 BS EN ISO 11997-2:2013, “Paints and varnishes. Determination of resistance to cyclic corrosion conditions. Wet (salt fog)/dry/humidity/UV light” (London, U.K.: BSI, 2013).
6 ISO 4628-2:2016, “Paints and varnishes —Evaluation of degradation of coatings -- Designation of quantity and size of defects, and of intensity of uniform changes in appearance —Part 2: Assessment of degree of blistering” (Geneva, Switzerland: ISO, 2016).
7 ASTM D1141-98(2013), “Standard Practice for the Preparation of Substitute Ocean Water” (West Conshohocken, PA: ASTM, 2013).
8 ASTM D1653-03, “Standard Test Methods for Water Vapor Transmission of Organic Coating Films” (West Conshohocken, PA: ASTM, 2003).