Single-coat inorganic zinc (IOZ) silicate coatings can provide long-term protection to steel, especially in marine atmospheric environments. As a single coat, they allow rapid coating work. As a relatively thin coating, they are also very economical. Some problems can arise when using these coatings, however, which have led to their avoidance, misuse, or failure.
Does the Application of Inorganic Zinc Silicate Coatings Require Special Skills?
Although the skills required for successfully mixing and applying IOZ coatings can be easily learned, treating IOZ in the same way as other paint can result in faulty application. For example, zinc pigment is heavy and will rapidly settle out. It must be continuously agitated. The applicator must apply the coating as a wet coat in even, parallel passes. Thickness, temperature, and humidity conditions are all critical for proper curing. Skilled applicators are essential.
How do Inorganic Zinc Silicate Coatings Cure?
For the solvent-borne product, the first stage is evaporation of the solvent, which readily occurs even under very cold conditions. After evaporation of the solvent, alkyl (typically ethyl) silicate reacts with water (a hydrolysis reaction) to form a silanol and ethanol. The silanol molecules cross-link in a condensation reaction to form a silicate polymer and water. The zinc ions, produced by the addition of zinc dust to the silicate binder, serve to catalyze both the hydrolysis and condensation reactions.
Although commonly believed to react with atmospheric moisture, Eccleston1 has shown by gas chromatography that water for the hydrolysis reaction is, in fact, the water released from the condensation reaction. This is confirmed by practical observations. One manufacturer has recently introduced a humectant additive (the opposite of a desiccant) for dry conditions, which holds the condensation reaction moisture within the coating film while it cures. Moreover, some manufacturers allow application of their products down to 0 °F (–18 °C), even though there is no liquid water available in the atmosphere. Below the freezing point of water, the silicate liquid acts as an antifreeze so the two reactions can still proceed, albeit at a slow rate. Above 32 °F (0 °C), however, a humid environment is required to ensure that water remains within the uncured coating so the practical effect is the same as if the reaction occurred with atmospheric moisture.
Ideal conditions for application of a solvent-borne IOZ silicate coating are a surface temperature of 68 to 77 °F (20 to 25 °C), with a relative humidity (RH) between 70 and 90%. Curing times increase as actual conditions move further away from these ideal conditions. Under conditions of low humidity, the coating “dries” but a soft, friable, uncured coating results. Water misting or steaming must be carried out if the RH drops below 50% during curing (60% if overcoated), but such actions will not cure a coating that has dried. The coating also will cure under very high humidity conditions, but evaporation of the solvent will be slowed and there is greater risk of damage from rain or condensation. There are a number of tests that have been suggested for checking the cure of solvent-borne IOZ silicate coatings, but an investigation by Starr2 found that the methyl ethyl ketone (MEK) solvent rub test (ASTM D47523) was the only reliable field method.
For the water-borne product, the first curing stage is evaporation of the water, so warm, dry conditions are required. After the water evaporates, one of the first reaction products during the cure is an alkaline hydroxide (OH–) (typically potassium hydroxide [KOH]). Unlike the ethanol that forms during cure of the solvent-borne product, the hydroxide remains on the surface. Under normal conditions, this will neutralize through a reaction with atmospheric carbon dioxide (CO2) and moisture. However, heavy dew or water ponding can cause this hydroxide to attack the zinc or unreacted silicate and cause spot rusting, blistering, or a soft, uncured film (Figure 1).4 Heavy rain will usually wash the hydroxide out of the film, so this damage might not arise. Depending on the weather conditions, it may be weeks or months before the hydroxide is reacted or removed from the coating and a neutral surface is achieved.

Ideal conditions for the application of water-borne IOZ silicate coatings are a surface temperature of 68 to 77 °F (20 to 25 °C) and an RH between 40 and 50%. Under these conditions, curing to water resistance can be achieved in 2 or 3 h, although high-ratio types can reach water insolubility within an hour. Moving further away from the ideal conditions lengthens the coatings’ curing time. Water-borne products should not be applied below 40 °F (5 °C), and high temperatures may result in dry spray. The coating must be protected from rain or heavy dew during the curing period. Although not often indicated, moving air is also important for drying any water-borne coating; and use of fans to avoid stagnant air conditions is strongly recommended.5
To prevent an applicator from being forced to apply an IOZ silicate coating under climatic conditions where it is not likely to achieve a satisfactory cure, provisions should be made by specifiers to allow either a solvent- or water-borne silicate coating to be used depending on prevailing conditions.
What Problems Arise with Excessive Thickness?
One of the problems with IOZ silicate coatings, especially solvent-borne, is their tendency to mudcrack when applied too thickly; but mudcracking is not as serious as it may appear. With many formulations, it does not become visible unless the coating is hundreds of microns thick.6 For trained and experienced applicators, keeping the thickness at appropriate levels should not be a problem. The corrosion products normally fill in the cracks in the same way that fine cracks in concrete will often self-heal. A thorough visual inspection should be carried out to ensure there is no severe mudcracking, which requires repair. Minor mudcracking that is adherent usually can be left without serious consequences.
Szokolik7 notes that excessive thickness can adversely affect the cure of high-ratio, water-based IOZ silicate coatings applied in less than favorable ambient conditions and suggests a maximum thickness of 6 mils (150 µm) for these materials. This would probably be a reasonable upper limit for any IOZ coating. It is more critical, however, to ensure there is sufficient coating covering every part of the structure. To achieve this, the target film thickness should eliminate the chance there will be regions with less than 3 mils (~75 µm) of coating. To achieve this, skilled applicators should aim for a minimum of <~4 mils (100 µm). This should ensure there is sufficient coating over the entire surface to minimize the risk of premature breakdown in regions with low dry film thickness (DFT).
Can an Inorganic Zinc Silicate Coating Be Repaired with Itself?
Repair recommendations for IOZ silicate coatings can range from complete removal to repair with epoxy zinc or surface-tolerant epoxy coatings.8 In fact, with a few extra considerations that differ from the repair of other coating types, an IOZ silicate coating can be recoated with itself, although this requires skill and care.
If the coating is found to be too thin after application and an extra coat is required, the silicate solution from any repair coat will migrate into the voids and leave a friable and powdery surface if the surface porosity has not had a chance to fill with corrosion products. The liquid content of the repair coat should be increased by 10 to 15% so there is sufficient liquid to prevent the surface layer from becoming underbound and lacking in adhesion.
Once the coating has aged, porosity is no longer an issue. As with any other repair coating, the surface must be properly cleaned before applying a topcoat of IOZ. As long as the surface is thoroughly clean and free from loose contamination, it will be suitable for overcoating. An IOZ coating can be repaired with either solvent-borne or water-borne IOZ silicate coating, which will perform better than alternatives. The solvent-borne IOZ has better wetting characteristics and is easier to apply in the field, and therefore is recommended as long as humidity conditions are suitable. The initial adhesion immediately after application of a repair IOZ coating is poor,6 but once the repair coating is thoroughly cured (which may take several months), an adherent bond develops between the new and old coatings, as shown in Figure 2.

Does a Topcoat Provide Additional Protection to a Single-Coat Inorganic Zinc Silicate Coating?
More coats are usually better for conventional coatings, but IOZ silicate coatings behave differently. As shown in the diagram in Figure 3, applying a topcoat prevents the formation of protective corrosion products. Furthermore, the cathodic protection for bare areas of steel is reduced by a topcoat as it considerably reduces the available area of the anodic zinc. Many tests have shown that topcoating is not necessary and, in some cases, can actually be detrimental to the coating system’s durability.9 Topcoats should only be applied if color is required, and then an epoxy zinc primer system should be specified that is easy to apply, has fewer drying and curing restrictions, and provides similar performance as a multi-coat system.10
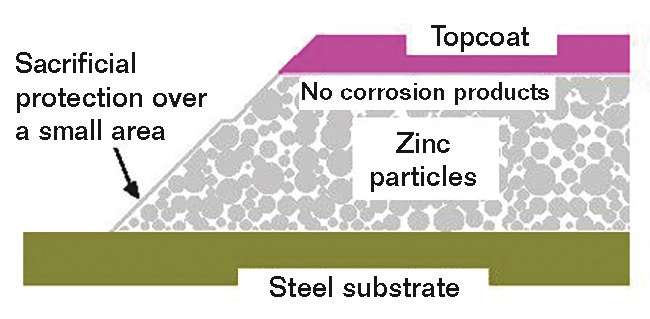
What Factors Must be Considered if Topcoating Inorganic Zinc Is Necessary?
Topcoating adds to the concerns and activities during application. Curing requirements for multi-coat systems are even more critical than for single-coat systems because once the topcoat is applied, further curing of the IOZ coating cannot take place. When applied to uncured zinc, topcoats can fail by zinc splitting.
The porous nature of the zinc can cause bubbling (gassing) or pinholing of the topcoat, which usually results in a cratered appearance when dry (Figure 4). There are a number of techniques used to minimize this risk, including use of a low DFT mist coat, application of an extra topcoat that is thinned down considerably, or use of a compatible tie coat.11 Ideally, the topcoat should be applied after the IOZ has been exposed to the weather for several weeks, and these problems are rare when topcoating shop-primed steel on site after construction.

Water-borne IOZ coatings normally should not be topcoated because of the additional problem of possible residual alkalinity, which can destroy any applied coating. However, a water-borne IOZ silicate coating topcoated with acrylic latex is a unique system that combines excellent durability with low or no volatile organic compounds (VOCs), and can be used as a “green” coating system where color and good durability are required. This system was used when the iconic Golden Gate Bridge that crosses the San Francisco Bay was repainted in the 1980s.
If this system is specified, it is essential that there is no residual alkalinity on the primer surface in addition to all the other concerns regarding drying, curing, and topcoating. Investigations by the U.S. Department of Transportation12 showed acrylic topcoats over water-borne IOZ coatings inevitably blistered within 60 days unless the primer was neutralized. These researchers recommended testing the pH of the surface if it is to be overcoated, to ensure it is between 4 and 7.
Conclusions
IOZ silicate coatings, whether solvent-borne or water-borne, provide a number of unique challenges for the specifier and applicator. However, the excellent protection, rapid application, and good economy provided by these systems suggest it is worth learning to apply them properly and understanding their limits and idiosyncrasies. Successful application will reward the asset owner with a unique and powerful product in the fight against corrosion.
References
1 G. Eccleston, “Effect of Cure Temperature and Humidity on the Properties of Solvent-Borne Zinc Silicate Coatings,” JPCL-PMC Jan (1998): pp. 36-45.
2 T.L. Starr, “Improving the Reliability of Zinc- Rich Paint Systems,” JPCL Vol. 3 (3) (1986): pp. 22-31.
3 ASTM D4752–10 (2015), “Standard Practice for Measuring MEK Resistance of Ethyl Silicate (Inorganic) Zinc-Rich Primers by Solvent Rub” (West Conshohocken, PA: ASTM, 2015).
4 W.L. Mandeno, correspondence to author, March 14, 2013.
5 “Problem Solving Forum,” JPCL Oct (1995): p. 11.
6 R. Francis, et al., Corrosion & Materials Jun (2008): pp. 30-37.
7 A. Szokolik, “Field Testing of Inorganic Zinc Silicates,” JPCL May (1995): pp. 56-67.
8 “Problem Solving Forum,” JPCL Nov (1998): p. 11.
9 W.J. Paton, “Performance Characteristics of Zinc-Rich Coatings Applied to Carbon Steel,” NASA Technical Note, D-7336, July 1973.
10 G.R. Ruschau, et al., “Specifications vs. Reality for Selection of Protective Coatings,” CORROSION 2008, paper no. 017 (Houston, TX: NACE International, 2008).
11 SSPC-PS Guide 8.00, “Guide to Topcoating Zinc-Rich Primers” (Pittsburgh, PA: SSPC, 2002).
12 J.P. Ault, C.L. Farschon, “Adhesion Criteria Between Water-Based Inorganic Zinc Coatings and Their Topcoats for Steel,” Federal Highway Administration, FHWA-RD-98-170, March 1999.