Hexavalent chromium [Cr(VI)] has been used for many years as an anticorrosive agent in protective surface coatings for aircraft. Primers containing Cr(VI) have been used in coating systems applied to U.S. Department of Defense (DoD) military aircraft to shield aluminum from corrosion. Airframe structural components that are comprised of aluminum alloys—such as AA 2024-T3 (UNS A92024) for panels and AA7050-T74511 (UNS A97050) for flap hinges, trailing edge structures, and missile ribs—are particularly prone to corrosion because seawater can collect in these areas.
In primer coats, Cr(VI) is present in the form of strontium chromate, and inhalation exposures can occur when workers apply the Cr(VI)-containing primer to the metal surface. In addition, mechanical abrasion of aircraft surfaces can generate particulates that contain chromates from previously applied Cr(VI)-containing primers.1 Because of concerns over the toxicity of Cr(VI) and its link to cancer,2 alternative non-chromated coatings have been sought for corrosion protection.
“Historically, across the DoD and even in commercial aerospace, chromated primers have been the main game in terms of protective primer applications, and they work extremely well,” says NACE International member Adam Goff, senior research scientist and team leader—protective materials with Luna Innovations (Charlottesville, Virginia). “Since increased toxicity concerns with chromated systems have come to light, there’s been a major effort over the past several years to develop, characterize, and qualify the performance of non-chromated primers and non-chrome-containing protective coatings.”
While numerous non-chrome primer technologies exist across the aerospace industry, none offer superior performance to traditional chromated primers and only a few utilize the beneficial attributes of carbon nanomaterials. Scientists at Luna Innovations have developed one such non-chrome primer system by incorporating carbon nanotubes (CNTs) modified with organic inhibitors into an epoxy-based resin. The goal of the coating development effort was to identify promising CNT-containing non-chrome primer formulations that perform comparably to traditional chromated ones. CNTs, cylindrical nanostructures that are a structural modification of carbon, have previously been used without inhibitors in coatings for corrosion protection of aluminum, and deliver an improvement in the coatings’ mechanical properties (increased hardness and crack and abrasion resistance) and barrier properties (reduced diffusion of moisture, salt, and other corrosive species). The CNTs enhance the corrosion resistance of the coating by making it more durable and resistant to water and chloride ingress. The addition of thiazole derivatives—the organic inhibitors used by the scientists—passivates the metal surface and also further restricts the movement of corrosive species through the coating.
“We’ve done work in the area of non-chrome inhibitor development and characterization, and a lot of work, separately, with carbon nanomaterials, but we hadn’t really combined the two,” says Goff. For this development, the scientists joined the two technologies by binding commercial off-the-shelf corrosion inhibitors to the surfaces of multi-walled CNTs. When added to the epoxy-based primer resin system, the modified CNTs improve the coating’s mechanical and barrier properties, as well as serve as a carrier to disperse the corrosion inhibitor throughout the coating.
Goff explains that two binding processes—covalent and noncovalent—attach the organic inhibitors to the surfaces of the CNTs. “What we’re doing, which is unique, is physically and chemically attaching the additive to the carbon nanotubes in a modification process,” he says. The two binding mechanisms that attach the inhibitor to the modified CNTs enable comprehensive corrosion inhibition when the CNTs are incorporated into a primer coating.
Through a reaction with the carboxylic acid groups on the CNT’s surface, the organic corrosion inhibitor molecules are chemically (covalently) attached to the nanotubes. As these tightly adhered inhibitor molecules are dispersed throughout the coating as part of the CNTs, they block the mobility of corrosive ions through the coating, as well as provide local corrosion inhibition at the coating/aluminum interface. Excess inhibitor molecules that are not chemically bound to the CNTs are physically (noncovalent) bound to them through surface adsorption, which enables the inhibitor molecules to separate from the nanotube structure and absorb into the aluminum surface during coating application and also migrate to the coating/aluminum interface at defects and damage sites. The advantage of having both inhibitor attachment mechanisms present in the primer, Goff comments, is that they provide an effective means of enabling both immediate aluminum substrate protection during coating application as well as long-term protection associated with covalently attached inhibitor molecules.
The CNT-infused epoxy primer coating is designed to be applied at 1 to 2 mils (25 to 50 µm) dry film thickness, the same thickness as traditional chromate primers. Goff notes that scientists achieved an optimal loading level of CNTs, which facilitates the coating’s mechanical improvement and enhanced corrosion inhibition while still maintaining high electrical resistance. To test its corrosion performance, the fully formulated CNT primer was spray-applied to 3 by 6 in (76 by 152 mm) UNS A92024 aluminum alloy sample panels pretreated with a chromated conversion coating. The coated panels were cured for seven days under ambient laboratory conditions prior to testing.
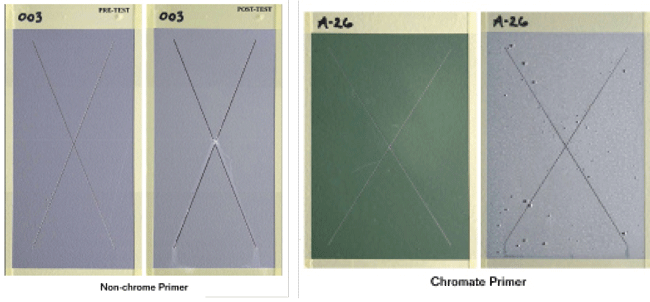
The panels were then mill scribed with an “X” through the coating down to the aluminum substrate and placed into ASTM B1173 neutral salt fog corrosion resistance testing for 2,000 h. As a control, a conventional chromated primer was tested on identically treated aluminum panels. At the conclusion of the test, the panels were visually examined, and the CNT chrome-free primer showed no signs of blistering, softening, lifting, or any corrosion extending more than 0.03 in (0.76 mm) beyond the scribe lines. Also, no corrosion creep was observed along the scribe. Slight formation of some corrosion product was observed within the scribe; however, the amount was similar to that observed in the chromated control primer. Overall, the modified CNT chrome-free primer demonstrated excellent corrosion resistance to neutral salt spray with performance equivalent to that of the chromated control primer.
The CNT chrome-free primer coated panels also performed well during exposure to cyclic sulfur dioxide (SO2) salt spray for 500 h according to Annex A4 in ASTM G85.4 A conventional chromated primer was also tested on identically treated aluminum panels as a control system. Results indicated the corrosion performance of the modified CNT primer exceeded that of the chromated control primer in ASTM G85-Annex A4 salt spray exposure. These results indicate that the modified CNT chrome-free primer is a viable alternative to Cr(VI)-containing primers with the potential of providing enhanced corrosion mitigating performance.
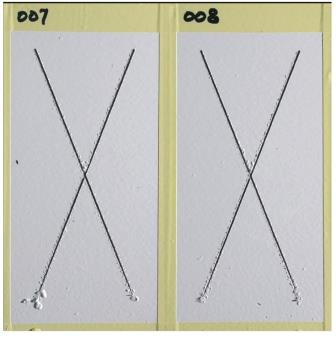
The susceptibility of “X” scribed conversion coated aluminum panels to filiform corrosion (fine, thread-like lines [filaments] of corrosion products under a coating that lead to flaking and bubbling) when coated with the CNT chrome-free primer and a polyurethane topcoat was tested in accordance with ASTM D2803.5 The panels were exposed for 1 h in a closed desiccator containing 750 mL of hydrochloric acid (HCl), and then exposed for 30 days at 104 °C and 85% relative humidity. The primer passed the test, with the longest filament measuring 0.094-in (2.38-mm) long, and the majority of the filaments measuring between 0.016- and 0.063-in (0.4- and 1.6-mm) long. Additionally, the CNT chrome-free primer achieved all requirements necessary to meet MIL-PRF-23377K.6
The next step, Goff says, is to prepare larger-scale batches of the CNT chrome-free primer to demonstrate the coating formulation’s repeatability, and then work with a toll manufacturing partner to produce a quantity suitable for aircraft patch testing. Currently, he adds, the scientists are actively working with Naval Air Systems Command (NAVAIR) to identify an aircraft for applying the non-chromate CNT primer in a test area to determine its performance during actual atmospheric exposure. In addition, coated panels are undergoing beachside exposure testing at the Kennedy Space Center, Florida.
Research for the non-chromate CNT primer was funded through a DoD Small Business Innovation Research (SBIR) Phase II grant for the U.S. Navy, contract no. N68335-13-C-0070, “Long Range Ion Exchange for Non-Chrome Corrosion Inhibition.”
This article is based on CORROSION 2015 paper no. 5797, “Non-Chrome Corrosion Resistant Primers with Carbon Nanotubes for Improved Performance,” by R. Jeffers, A. Goff, J. Garrett, and C. Sprinkle.
Contact Adam Goff, Luna Innovations— e-mail: goffa@lunainc.com.
References
1 G.N. Carlton, “Hexavalent Chromium Exposures During Full-Aircraft Corrosion Control,” AIHA J 64, 5 (2003): pp. 668-72.
2 “Hexavalent Chromium,” Safety and Health Topics, U.S. Department of Labor, Occupational Safety & Health Administration, https://www.osha.gov/SLTC/hexavalentchromium/ (December 3, 2015).
3 ASTM B117-11, “Standard Practice for Operating Salt Spray (Fog) Apparatus” (West Conshohocken, PA: ASTM, 2011).
4 ASTM G85-11, “Standard Practice for Modified Salt Spray (Fog) Testing” (West Conshohocken, PA: ASTM, 2011).
5 ASTM D2803-09 (2015), “Standard Guide for Testing Filiform Corrosion Resistance of Organic Coatings on Metal” (West Conshohocken, PA: ASTM, 2015).
6 MIL-PRF-23377K, “Performance Specification: Primer Coatings: Epoxy, High-Solids” (Washington, DC: MIL, 2012).