For this article, graphene modified with amines was prepared. The performance of different graphene oxide (GO)-based corrosion inhibitors (CI) was investigated over a wide range of parameters. In the presence of low-CI concentrations (i.e., 25 ppm), the corrosion inhibition efficiency was found to be more than 85% in the presence of 15% hydrochloric acid (HCI) up to 194 °F (90 °C).
The inhibition efficiency decreased significantly when the temperature was increased. The preparation of GO-based nanomaterials from graphite has been detailed in this study, and the performance of these materials was evaluated as CI in the presence of different acid systems.
NOTE: This article was adapted from “Recent Advancements of Corrosion Inhibitors Using Graphene OxideBased Nanomaterial.”1
Corrosion is a spontaneous chemical reaction connected to heat exchangers and pipelines that degrades the strength and quality of utilized steel.2 The ongoing aggressive environment, which is intense and frequently difficult to prevent, is the key factor contributing to the metal’s deterioration.3
The use of corrosion-resistant alloys, anti-corrosion coatings, corrosion inhibitors (CI), cathodic protection, and anodic passivation are just a few examples of the anti-corrosion techniques that are widely used in the manufacturing process.4–5
Because stainless steel has a high amount of chromium metal (Cr 15% to 20%), it is the most frequently used steel for corrosion-resistant materials.6–10 However, harsh environments like saline and acidic media tend to produce a significant amount of corrosion damage on the surface of many stainless steels. As a result, a wide variety of corrosion-resistant compounds, including inorganic and organic-based materials, have been developed for reducing corrosion.11
Graphene is utilized as a material for anti-corrosion coatings because it cannot dissolve in water and therefore lacks soluble functional groups.12 The effectiveness of graphene can be increased through surface modification using different functional groups.
Covalent functionalization of graphene oxide (GO) might be accomplished using the carboxylic acid groups on the edges, the epoxy/hydroxyl groups on the basal plane, and the functional oxygen groups on the surface. Due to the numerous oxygen-containing functional groups—including epoxides, hydroxyls, carbonyls, and carboxyls—that are present on their surfaces and edges, GO and functionalized GOs may function as effective CI in the solution phase.13
For this article, GO was synthesized and modified with diaminodecane and diaminododecane. The obtained inhibitors were then evaluated for corrosion inhibition.
Experimental Procedure
Materials
Potassium permanganate (KMnO4), sulfuric acid (H2SO4) with purity as 96% w/w, phosphoric acid (orthophosphoric acid, monophosphoric acid, or phosphoric [V] acid) (H3PO4) (85% w/w), hydrogen peroxide (H2O2) (30%), diaminodecane (NH2[CH2]10NH2), and diaminododecane (NH2[CH2]12NH2), were from Sigma Aldrich in the USA.
API X60 carbon steel was purchased from Metal Samples Company in the USA.
Synthesis Procedures
The GO was created using discarded graphite powder. In brief, 360 ml of ice-cold H2SO4 (96%) and 40 ml of H3PO4 were stirred, and then a combination of 3g of graphite powder and 18g of KMnO4 was slowly added.
After being heated for 12 h at 122 °F (50 °C) while being stirred, the liquid was allowed to cool at room temperature for an additional night before being put into 400 ml deionized water ice with 3 ml of H2O2 (30%) added. Before the supernatant was taken out, the finished product was left to settle overnight.
After that, it was repeatedly washed in water to remove any leftover acid. After three rounds of washing with 10% HCl and distilled water to remove unreacted metal ions, the remaining material was finally mixed in deionized water, where unreacted graphite precipitated and the GO dissolved.
The dissolved GO was decanted, spun at 1,000 rpm for 1 h, and then dried. Next, 200-ml aliquots of the resultant GO solution were ultrasonically processed at 500 ppm for 30 min in anhydrous dimethylformamide before 0.3 g each of diaminodecane and diaminododecane were added.
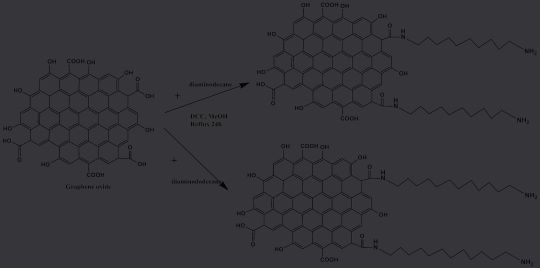
The mixtures were mixed for 24 h at room temperature before being centrifuged, repeatedly washed in a 1:1 mixture of ethanol and water, and then dried at 149 °F (65 °C). Figure 1 shows an example of how to prepare GO with diaminodecane functionalization (DAD-GO) and diaminododecane functionalization (DADD-GO).
Weight Loss Measurement Experiment
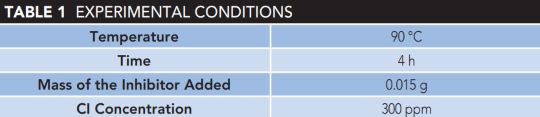
The ASTM G1-03 methodology was used for the weight-loss method. Preweighed carbon steel specimens were immersed entirely in duplicates in 100 ml of the test solutions housed in a 250 ml glass container held at high temperatures for 24 h. After each test sample was thoroughly cleaned, rinsed with distilled water and acetone, dried, and weighed, it was removed.
The weight loss was calculated using the difference between the samples’ initial and final weights, and the corrosion rate was calculated using the average weight loss, as shown in Table 1.
Results
Characterization
Figure 2 (top) depicts the transmission electron micrograph (TEM) of the diaminododecane-modified graphene. The TEM pictures reveal the flake-like form and wrinkled features typical of GO sheets.
At increased magnification, GO’s folded and crumpled shape, which is connected to the oxygen-containing functional groups on the surface of GO,14 could be made out. The folded and crumpled morphology is more obvious in the GO modified with diaminodecane and diaminododecane. This results from the amino functional groups of the functionalized GO forming hydrogen bonds with one another.15
Weight-Loss Measurement
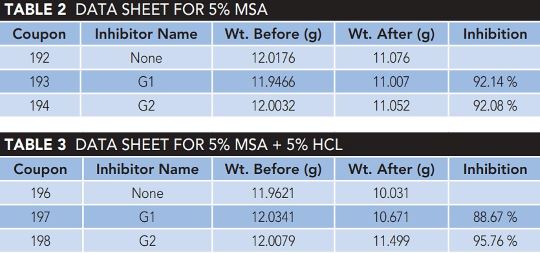
Table 2 displays the weight-loss findings for GO treated with diaminodecane and diaminododecane test solutions at high temperatures.
The corrosion rate was calculated using Equation 1, where W refers to the average weight loss in grams (g), A refers to the total exposed surface area in square centimeters (cm2), T refers to immersion time in h, and D refers to coupon density in grams per cubic meter (g/cm3).
Percent inhibition efficiency or %IE was calculated using Equation 2. CRo and CRI are the corrosion rates for the blank solution and inhibited test solution, respectively. The modified GO-inhibited solution corroded at a slower rate than the blank.
Exfoliated GO nanosheets tend to stay together by π to π stacking, generating large graphite oxide nanosheets. The grafting of diaminoalkanes on the GO surface stabilizes the GO nanosheets, improving GO performance as indicated by the modified GO, as shown in Table 3, having a lower rate of corrosion than the blank. The corrosion rate values for both modified GO-inhibited solutions were lower than blank.
The inhibitor can be adsorbed on metal or steel surfaces through electrostatic attraction/physical mechanism (physisorption) when the protonated inhibitor molecule has an interaction with adsorbed negative chloride ion or organic species on the metal surface.
Chemical mechanism (chemisorption) can be undergone when the heteroatom in inhibitor molecules donate lon e pair/π-electrons from O (oxygen atom) to the vacant 3d-orbitals of (Fe) metal of the steel.15
Conclusions
The study used diaminodecane and diaminododecane functionalized GO to mitigate carbon steel corrosion in acid solutions (simulate oil well acidizing environment). Graphite powder was used to create the GO.
The results showed that the diaminodecane- and diaminododecanemodified GO demonstrated high inhibitory efficiency at high temperatures. Because the GO was created from scrap graphite, this work offers the added benefit of solving both corrosion and waste disposal issues.
Editor’s note: This article first appeared in the July 2024 print issue of Materials Performance (MP) Magazine. Reprinted with permission.