In this study, a piping failure during fire water service operation is investigated using metallographic techniques. Metallurgical investigation as a part of an inspection program in petroleum and petrochemical plants is useful to find out the root causes of corrosion.
The results showed that standard welding procedures were not followed, leading to undesired size variation of grains in the microstructure. The fire water was used without any pre-treatment and probably contained foreign elements like chlorine, sulfate, and silica that were responsible for corrosion in piping. Based on collected evidence, some practical recommendations were suggested for fire water service applications.
In many cases, carbon steel (CS) is used as a material of construction for piping that transports fire water, cooling water, and recycled water in major industrial complexes. Many leaks have been noted in those piping systems after several years of utilization.
Therefore, fire water piping undergoes major repair activities in addition to complete replacement. The fire water piping may fail to perform during emergency fires and explosions.1
Many publications have reported on various issues associated with failure of water and other similar piping and tubes.1-8 It is established that internal uniform and pitting corrosion are common in industrial utility piping systems made from CS.2-5 Furthermore, a large number of failures were observed in weld joints due to the variation of microstructure in response to corrosion.4
The fire water employs water service without any water pre-treatment and therefore, foreign element contamination severely affects the water properties. Few investigation reports clearly show that natural water contains chloride and sulfate, which should be precluded before utilization.3
The chloride and sulfur ions accompanied with other elements increase both uniform and localized corrosion of the piping system. Therefore, piping maintenance is extremely important, especially for hydrocarbon industries. This article will provide brief information on a CS piping failure at a weld joint in addition to field analysis of fire water used in a complex petroleum industrial complex.
Experimental Methods
Case Definition
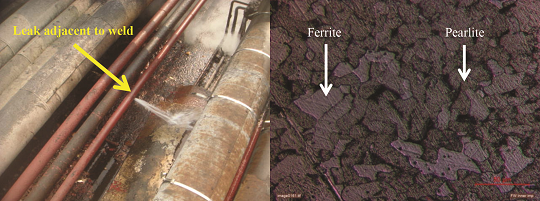
The failed pipe was 100 in (2,540 mm) in diameter with a thickness of 6.02 mm and a corrosion allowance of 1.5 mm operated at atmospheric pressure and temperature [Figure 1(a)]. During construction, the pipes were welded, with the edges prepared with a single V groove butt joint and subsequently root welded with CS (E70S2) filler wire using tungsten inert gas welding.
Further passes were welded with CS (E-6018) electrode using shielded metal arc welding and subjected to nondestructive tests before operation. The chemical composition of the base metal pipe and weldment measured by x-ray fluorescence are shown in Table 1.
The fire water is directly pumped from a nearby lake and has been used in refinery fire water service without any pretreatment. The fluid remains stagnant except during flushing operations. The pipe leaked at several weld locations after 10 years of service, and a failed section was metallurgically analyzed.
Methodology
The failed weldment section of the sample was collected from the field and metallographically prepared to find the root cause of the failure. The sample was sectioned by cold cutting using a Spruers Discotom 65†. Further, it was mounted using automatic mounting press Buehler Simplimet 1000†. Subsequently, the sample was grinded and polished using Buehler Metaserv 250†.
The sample was fine polished to 0.25 µm without scratchfree surfaces and examined using optical microscopy using a Carl Zeiss Axio vert V1†. The fire water sample was collected and chemical constituents along with certain physical properties were measured.3,5
Postmortem Results and Discussion
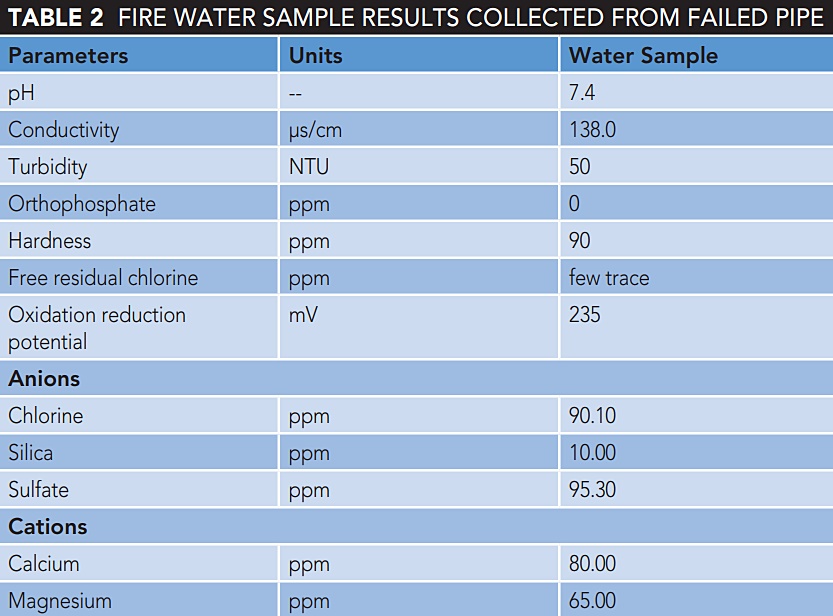
The base metal pipe was subjected to thickness gauging, which reveals significant reduction in wall thickness and metallography shows ferrite and pearlite microstructure [Figure 1(b)]. Both the weldment and heat affected zone (HAZ) consist of ferrite and pearlite microstructure [Figures 2(a) and (b)]. Also, the base metal and weld metal show significant variation in grain size and number of phases (Figure 2, top).
According to reference 3, the ferrite can act as the anode and pearlite can act as thecathode while fire water acts as the electrolyte forming an overall electrochemical reaction. The water deposits enriched in chloride and sulfate can alter localized chemistry under those deposits in the piping, thereby altering the localized pH that in turn will produce pits (Table 2).
The localized pH within the pit, however, was not measured but overall water chemical analysis along with thickness gauging confirmed the corrosion. The initiated pit has an auto catalytic effect to grow continuously until full penetration and form a leak.
Further, a large cathode exists and when coupled to a small anode can lead it to corrode at a fast rate. The grain size and amount of each phase present in microstructure have a profound effect on corrosion and therefore, control of grain size is also equally important. Fine grain size will increase the corrosion rate in comparison to coarse grain size, especially to normal industrial water corrosion.
Significant variation in grain size can be controlled by post-weld heat treatment, which can reduce the area effect for electrochemical reaction. Uniform grain size could reduce the corrosion rate compared to nonuniform grain size.
The metallographic evidence also shows that during construction the weld adjacent to the pipe had rapid cooling and to some extent attributed to small grain formation. The localized crack was evidenced in pearlitic structure of 1 mm and a few cracks were less than 500 µm [Figure 2(c)]. The weldment revealed a larger amount of pearlite phase than the base metal because of higher manganese constituents in the filler wire/electrode than the base pipe.
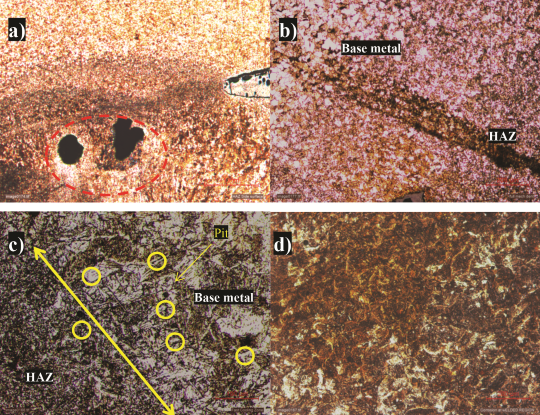
The large pits were initiated, and the chlorides accompanied with other foreign elements like sulfate in the water probably attributed to pitting corrosion adjacent to weld [Figures 2(c) and 3(a)]. Chidambaram3 demonstrated that chloride deposited at the 6 o’clock pipe position has resulted in grain boundary attack and acted as an anode while the grain interior acted as a cathode for API 5L Grade A line pipe steel.
The ferrite phase preferentially corrodes over the pearlite and the continuous presence of contaminated stagnant water attributed to complete rupture of weld joints that resulted in leakage [Figure 3(b)].
The fast cooling or an improper cooling gradient during initial construction probably attributed to crack initiation by thermal stress and cracks propagated to reach the critical size with delayed time, which was observed in the ferrite and pearlite phases [Figure 2(c)].
Interfaces were more prone to pitting attack due to anode and cathode formation [Figure 3(c)], while contaminant constituents in the fire water act as an electrolyte to form an overall electrochemical reaction at a localized area. Ferrite and pearlite phases were completely corroded [Figure 3(d)], revealing uniform corrosion of piping.
The recycled water and other waste water generated from the petroleum refinery were also pumped into fire water services, which further increases the corrosion rate due to the presence of significant foreign elements. We found similar water results in our earlier paper but some variations were observed due to continuous process upsets.
The following recommendations were proposed to avoid reoccurrence of similar failures, particularly for fire water services: chlorides and sulfate should be removed by proper chemical treatment and subsequently treated water should be used for fire water services. Proper welding should be done on industrial piping systems and fast cooling should be avoided on the weldment and HAZ area.
After welding, the weld joints should be subjected to radiography to identify cracks and other weld defects. CS pipe with an internal protective coating should be used for fire water service applications.9 Corrosion inhibitors should be added to intake water and its pH must be controlled.10
Conclusion
Inappropriate gradient cooling leads to cracks in the HAZ and base metal interfaces, resulting in significant grain size of ferrite and pearlite phases. Further, the contaminated water that contains chloride, sulfate, and other foreign elements has aggravated the initiation of pits, which grow continuously due to the electrochemical reaction.