Microbiologically influenced corrosion (MIC) is a significant corrosion problem resulting from the influence of microorganisms with other abiotic conditions and processes.1 While the mere presence of microorganisms in biofilms on metal surfaces does not always result in MIC, surface attached biofilms mediate MIC through various metal oxidation reactions.
It is therefore important to understand the characteristics of the microbial community as well as the resulting corrosion severity when investigating the threat of MIC and evaluating the performance of biocides.2
MIC Control and Why?
Fresh water, seawater, or brackish water from various sources may be introduced into pipelines, tanks, and other facilities as a part of hydrotesting and longterm layup. In addition to microorganisms, water provides nutrients necessary for biofilm growth and corrosion and hence, increases the likelihood of MIC during or after hydrotesting.
Microorganisms introduced during hydrotesting can remain in the pipeline during normal operations if not adequately treated during dewatering and cleaning. If left uncontrolled, MIC can ultimately result in leaks and ruptures of pipelines and tanks.3-4 Therefore, it is important to evaluate the threat of MIC during hydrotesting or wet parking and design appropriate mitigation methods to reduce the potential for MIC.
Before prevention or mitigation methods are implemented, MIC should be established as a relevant corrosion mechanism based on the source of the water, the conditions in the pipeline or tank before testing or layup, length of exposure time to water, and the nature of asset operation. In this case, evaluation of MIC control measures for a particular application included the following steps:
1. Assessment of MIC threat
2. Selection of MIC mitigation methods
3. Monitoring of MIC mitigation methods
Current Gaps in MIC Control
The threat of MIC in oil and gas assets has historically been evaluated using only culture-based tests that estimate a small subset of microbial functional groups related to MIC, such as sulfate-reducing bacteria, acid-producing bacteria, and general heterotrophic bacteria.5 There are several other microbial functional groups, including sulfate-reducing archaea, methanogens, sulfur-oxidizing bacteria, iron-oxidizing bacteria, and iron-reducing bacteria, that are also known to be corrosive; however, not all of these groups are identified through culture methods.
Although there are typically significant differences between planktonic and sessile (surface attached) microbial communities in terms of microbial numbers and diversity, serial dilution tests for biocide evaluation have frequently been conducted using liquid samples that are poorly representative of biofilms. Further, the culture-based tests misrepresent the true microbial community and give false positives and false negatives.6
Culture-based tests only detect the presence of a few commonly known microbial groups capable of causing MIC, but do not determine whether these microorganisms are actually causing any observed corrosion. There is no direct correlation between microbial numbers based on culture testing and the existence of a MIC threat, and similarly, there is no direct correlation between microbial numbers and the need for applying biocide to prevent or mitigate MIC.
To address these drawbacks in MIC threat identification using culture-based tests, a laboratory test method was outlined to evaluate the potential for MIC and subsequently assess the effectiveness of biocides to inhibit biofilm growth and mitigate MIC. Industry best practices have also advanced such that today, more emphasis is being placed on using biofilms for biocide evaluations, rather than basing performance on planktonic kill studies; consistent with the guidance in TM0194-2014 Section 5.3.7
Highlighting industry interest in this subject, AMPP committee TM21495 under Standards Committee SC-22, Biodeterioration, is currently working to develop a new standard on laboratory evaluation of the effect of biocides on biofilms.
Laboratory Testing for MIC Control
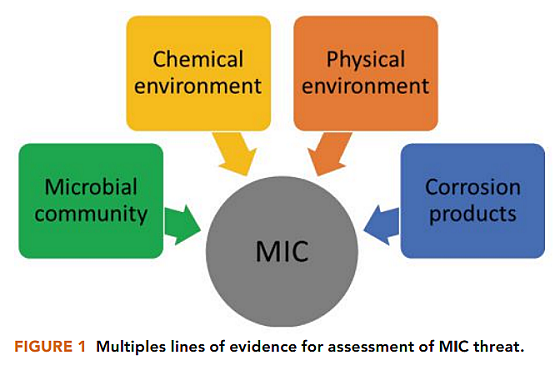
Because MIC is characterized by the interplay of microbial community, the chemical and physical environment, and corrosion products, it was important to use multiple lines of evidence to assess the threat of MIC (Figure 1).8 When investigating the extent of corrosion, it was also important to examine pit rates in addition to general corrosion rates, as MIC is most typically characterized by localized corrosion.
It is well known that systems with low rates of general corrosion can have high rates of pitting. If only general corrosion rates were monitored, information about pit rates and pit density would not be accounted for, resulting in erroneous MIC threat assessment and inaccurate monitoring of mitigation effectiveness.
As MIC is mediated by biofilms, it was essential to monitor biofilm populations in addition to planktonic populations, as significant differences between microbial abundance, microbial activity, and microbial diversity can exist between biofilms and the planktonic microbial populations.9-10 It was also important to analyze the water chemistry to identify compounds that support microbial metabolism and growth (such as sulfate, acetate, and organic compounds) as well as species that can promote abiotic corrosion (such as dissolved sulfides, dissolved oxygen, and chlorides).11
Monitoring for changes in the water chemistry revealed the flux of various chemical species, enabling further understanding of the corrosion mechanism. Evidence supporting abiotic corrosion mechanisms was weighed against evidence supporting MIC to identify the threat of MIC and further evaluate the effectiveness of mitigation methods for MIC.
A reliable method for MIC threat assessment and monitoring the effectiveness of MIC mitigation requires examining the impact of the biocide on biofilm growth and corrosion, either by the direct assessment of the corrosive environment in the field or by using process fluids to simulate the corrosive environment in the laboratory. A laboratory method for assessing the threat of MIC and monitoring the effectiveness of mitigation methods by mimicking field conditions using actual process fluids was developed and tested. The different mechanisms by which microorganisms and chemical species could affect corrosion and the laboratory simulation of natural or pipeline environments required forethought and careful experimental design. Conceptual design of a laboratory test method for MIC control is illustrated in Figure 2.
For MIC threat assessment, differentiation between abiotic and biotic corrosion can be accomplished by preparing two sets of experiments: one using the process fluids and the other using process fluids that have been sterilized (through autoclaving, ultraviolet light, filtration, or other appropriate methods). The changes to the biofilms and planktonic microbial populations (abundance, diversity, and activity), corrosion/pitting rates, and corrosion products can be monitored throughout the duration of the testing. Comparison and integration of evidence from these parameters between tests of the process fluids and the sterilized process fluids can help reveal the cause of corrosion.

For monitoring the effectiveness of mitigation in the lab, an untreated control can be used to compare the effectiveness of mitigation (chemical and/or concentration) on microorganisms, the chemical and physical environment, and the corrosion products that form. Based on the situation, the impact of the chemical addition can be determined, either on biofilm and MIC prevention or biofilm and MIC mitigation. For investigating biofilm and MIC prevention, chemically treated and untreated fluids can be incubated with metal coupons and the changes to the biofilms and planktonic microbial populations (abundance, diversity, and activity), corrosion/pitting rates, and corrosion products can be monitored throughout the duration of testing.
For investigating biofilm and MIC mitigation, process fluids can be first incubated with metal coupons and after an appropriate incubation time, the fluids can be treated with the chemical and the changes to the biofilms and planktonic microbial populations, corrosion/pitting rates, and corrosion products between the untreated and treated experiments can be monitored. Comparing the differences between these parameters of the treated and untreated experiments can help reveal the effectiveness of the mitigation.
Case Study
Laboratory tests were conducted on a water sample collected from an asset with documented corrosion issues to determine the corrosion mechanism and to evaluate the effectiveness of biocide treatment. Carbon steel coupons were exposed for four weeks to three conditions: 1) untreated water, 2) water with 100 ppm of biocide, and 3) water with 1,000 ppm of biocide. The changes to the biofilms and planktonic microbial populations, corrosion/pitting rates, corrosion morphology, and corrosion products were monitored throughout the four weeks of testing for these three conditions.
Black surface deposits formed of iron sulfide (FeS) were observed on coupons exposed to untreated water and water treated with 100 ppm of biocide. Biocide treatment reduced the biofilm activity in both treated samples but no significant difference in biofilm microbial abundance was noticed by the end of four weeks.
However, biofilms from the untreated reactor indicated the presence of several metabolic groups of microorganisms implicated in MIC (such as fermenters, methanogens, iron oxidizers, iron reducers, and denitrifying bacteria) while the treated reactors did not indicate the presence of these metabolic groups. Coupons exposed to untreated and 100 ppm biocide-treated waters showed corrosion rates up to four times higher than the coupons in 1,000 ppm biocide-treated water, indicating a correlation between the presence of the black deposits and the observed corrosion rates. However, severe pitting corrosion observed in the 1,000 ppm biocide-treated water highlighted the possibility of pit initiation and growth when the protective biocide film on the coupon surface became degraded. Characteristics of microscopic biotic pit initiation as well as signs of severe abiotic corrosion were observed on coupons from all reactors.
Integrating all the evidence collected during these tests, the probable cause for corrosion in the untreated and treated water was most likely abiotic corrosion with a minor component of MIC. The biocide treatment at 1,000 ppm concentration at first formed a semi-protective film on the coupon surfaces that perhaps helped initially in reducing corrosion rates and microbial activity and abundance, but then may have promoted severe pitting at places where the protective film was compromised as the residual biocide declined with time.
In this case, biocide application did not eliminate corrosion altogether because the water sample also indicated evidence of abiotic corrosion due to high sulfides and trace amounts of oxygen. The film-forming biocide may have resulted in more localized corrosion damage at places where the protective film was broken down over time and therefore, treatment chemicals with alternative modes of action could be considered to mitigate corrosion in this environment. For more details on this case study, please refer to our CORROSION 2021 paper.12
Summary
MIC control includes assessment of the MIC threat, selecting effective MIC mitigation, and monitoring of MIC mitigation effectiveness. A laboratory test framework for MIC control that considers multiple lines of evidence was described using a case study. The changes to the biofilm and planktonic microbial abundance, activity, and diversity; corrosion/pitting rates; and corrosion products were monitored before, during, and after the testing. Comparison and integration of information collected about these parameters revealed the cause of corrosion, guided the selection of an appropriate mitigation method, and measured the effectiveness of the mitigation applied under the test conditions.
References and About The Authors