Packaging is an industry term for the technology protecting any product intended for storage and shipping. Statistics indicate that the world market value for the packaging industry reached U.S. $1 trillion by 2020.1-4
During manufacturing, storage, and transport, humidity, salts, and other corrosive substances in the environment, as well as temperature fluctuations, can make metal parts more susceptible to corrosion. The likelihood of corrosion can be minimized either by controlling the environment or by insulating the metal from the destructive surroundings using suitable packaging materials containing corrosion inhibitors.
Anticorrosion packaging is an important trend in the packaging market because it provides optimum protection and extends the shelf life of the products. Due to their effectiveness, flexibility, low cost, and ease of application, the packaging technology finds its way into a wide range of products. Papers impregnated with volatile corrosion inhibitors (VCIs) have been used to protect sensitive electronic components, metals in various forms, automotive parts, machinery, engines, and tools.
The demand for VCI packaging has significantly increased; the VCI packaging market is expected to exceed U.S. $680 million by 2023, compared to U.S. $480 million in 2015 and U.S. $540 million in 2018. protection and extends the shelf life of the products. Due to their effectiveness, flexibility, low cost, and ease of application, the packaging technology finds its way into a wide range of products. Papers impregnated with volatile corrosion inhibitors (VCIs) have been used to protect sensitive electronic components, metals in various forms, automotive parts, machinery, engines, and tools. The demand for VCI packaging has significantly increased; the VCI packaging market is expected to exceed U.S. $680 million by 2023, compared to U.S. $480 million in 2015 and U.S. $540 million in 2018.
Corrosion is an undesirable electrochemical reaction for metals and alloys in response to an aggressive environment. A corrosion inhibitor is a chemical substance that, when added in small concentrations to an environment, can minimize or decelerate the corrosion process. Generally, an efficient inhibitor is compatible with the environment, is economical to use, and produces the desired effect when present in small concentrations.
In the past, a protective oil coating was used to prevent corrosion during transport and storage. The use of oil for corrosion protection involves its disadvantages. The oil coating must be removed before welding or painting. The removal of a protective oil coating also requires solvents. The disposal of used solvents has its own environmental restrictions. VCI packaging techniques facilitate corrosion protection of metal parts in light of the aforementioned complications.
VCI papers are highly effective and common packaging materials for protecting metals. They are cost-effective and easy to use for both ferrous and non-ferrous metals. These papers display good tensile strength and are mostly scratch/abrasion resistant. VCI papers are environmentally friendly, allowing for biodegradability and sustainability by recycling into other types of paper products, such as boxes and cardboard. Furthermore, they are void of hazardous ingredients, such as nitrites, phosphates, silicones, and chromates, or other heavy metals. VCI-coated papers are intended for wrapping metals during shipment, storage, and process operations. They are an effective means to protect dissimilar metals against galvanic corrosion.
VCIs are bio-based organic chemical compounds with significant vapor pressure, allowing vaporization of inhibiting molecules and further adsorption onto metallic surfaces. VCI molecules move from the paper directly to the surface of metal, and condense and adsorb onto the metal surface, forming an extremely thin molecular protective layer of crystals over the metal surface. This thin layer effectively inhibits corrosion on the metal by preventing air, moisture, salt, oxygen, and other corrosive elements from depositing on the metal surfaces.
In the presence of even trace amounts of corrosive substances, VCI molecules immediately develop strong bonding with the metal substrate. This layer separates the metal from the environment. VCI compounds are impregnated onto the packaging materials and released when they come into contact with the metal products within an enclosed space. Along with the corrosion protection, VCI packaging materials also act as moisture and dust barriers due to the hydrophobic nature of non-polar groups in organic VCI inhibitors.
VCIs protect both accessible and hard-to-reach surfaces of the metal from attack by corrosive species. VCI packaging does not change the properties of metal in any way and thereby, it has no effect on the electrical or mechanical properties of the protected material. After the metal part is taken out of the package, the VCI molecules volatize, and the metal is ready for immediate use, without any cleaning required.
Paper-based packaging materials possess low barrier properties against oxygen and water vapor penetration compared with plastic-based packaging materials.5-6 In this study, the principles of superhydrophobicity and the microstructure of kraft paper were used to control the VCI diffusion rate in the packaging papers. Superhydrophobicity can be created by embedding nanoparticles or by creating porosity. Thus, the inherent porosity of the papers was used to create semi-moisture barriers. Although the rough and porous surface of paper is necessary for creating high water contact angles, it also assists in the spreading and absorption of liquids through the fibrous geometry. The water contact angles of cellulose roughly vary between 17 and 47 degrees,3 while the range of the typical roughness of uncoated paper is within the range of 3 to 5 µm.7
The papers used in the experimental procedure are kraft papers, which consist of almost pure cellulose fibers. Cellulosic surfaces are comprised of hydrophilic fibers that make up a capillary system. Aspects, such as the capillarity and the hydrophilic nature of the cellulose fibers, increase the absorbency of the kraft paper. Inherently, kraft paper displays a rough and inhomogeneous surface, which draws itself from the intertwining and flocculation of the fibers in the slurry state from the paper formation. This non-uniformity is seen on the order of the fiber dimensions. The surface is actually an ill-defined boundary that depends on the degree of fiber compaction and collapse.8
Thus, the internal cavity of the cellulose fibers, surface roughness, and the pore networks are accountable for liquid or gaseous permeability in the paper. Moisture can migrate in the paper by a number of transport mechanisms: vapor-phase diffusion in the inter fiber pore space, Knudsen diffusion in pores of diameters <100 Å, surface diffusion over fiber surfaces, bulk-solid diffusion within fibers, and capillary transport.9
The movement of materials into and out of the paper takes place in either solution or gaseous forms. It is not clear which physical processes are involved; however, the movement is through imbibition and diffusion. Water flow in the fibrous layer moves along the fiber first and then fills the pore between them.10 The pores of the substrate were impregnated through spray-coating of the inhibiting solution. During the first spray, VCI compounds saturated the pores of the cellulose fiber, both inter and intra. The purpose of the second spray (superhydrophobic coating) allowed for the lowering of surface energy by altering the surface chemistry through silane derivatives, while further micro and sub-micro roughness was introduced. The increase of roughness adds to the tortuosity of both gaseous and liquid materials.
Hence, the moisture migration occurring by diffusion of water vapor through the void spaces and in condensed form through the fiber cell walls must now travel an increased path length.7,11 It is evident from the results that the micro-roughness arising from the position of the fibers in the network followed with a hydrophobization treatment hindered the vapor phase transport through the void spaces by increasing the tortuous paths.12
Experimental Procedure
The inhibiting ability of VCI coatings was evaluated by electrochemical polarization tests and vapor inhibiting ability (VIA) tests that were conducted before and after exposure to elevated temperature and controlled humidity (exhaustion tests). Visual examination of the metal surface was done using digital light microscopy and scanning electron microscopy to assess the corrosion grade for the steel specimen.
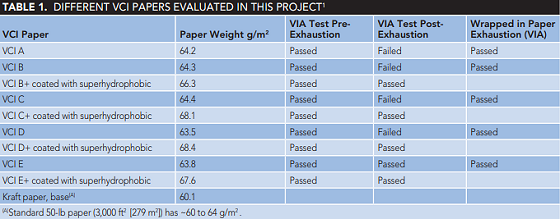
VCI A and B are two impregnated wrap papers commercially available. Additional VCI-coated papers were prepared by applying VCI C, D, and E on natural kraft paper (standard 50-lb kraft paper has a weight of ~60 to 64 g/m2) and drying the coated papers at 40 °C. One set of prepared VCI-coated papers was sprayed with hydrophobic moisture-repelling barrier coating after drying. Table 1 lists the different coated papers that were investigated. The vapor inhibiting ability of these VCI-impregnated wrap papers were investigated by using 2 by 8 in (5.08 by 20.32 cm) paper samples for the exhaustion tests. The exhaustion tests were conducted at 60 °C for 12 days as per MIL-STD-3010C13 Test Method 4031 for "Exhaustion Test of Packaging Materials."
During this test, air was maintained at ~50% relative humidity (RH) and pumped at a rate of ~100 cm3/min into the test tube. To achieve the proper RH, air passed through ~26% by volume mixture of glycerin/distilled water. Four exhaustion tests were conducted on each sample and after completion of the exhaustion test, the paper was cut to 1 by 6 in (2.54 by 15.24 cm) specimens for the VIA tests.
The corrosion behavior of carbon steel (CS) (UNS G10100) samples was evaluated per MIL-STD 3010C VIA test method and subjected to VIA tests (one control sample, three with impregnated paper, and six after exhaustion tests; 10 total samples for each VCI product). The VIA corrosion test method provides for standard conditions in a test jar of water-saturated warm air without the presence of any corrosive species. Prior to testing, all steel samples were prepared by polishing with 240, 320, 400, and 600 grit silicon carbide papers and a final polish with 1 µm powder. The VIA tests require: 1) sample conditioning for 20 h at 22 °C; 2) cooling cycle at 2 °C; 3) prewarming at 50 °C; and 4) followed by 3 h more at 22 °C. After this process, the steel samples were inspected for visible water condensation and the surface of each was visually examined by microscope to determine its corrosion rating.
The corrosion criteria for rating steel specimens consist of grade 0 (worst case, heavy corrosion) through grade 4 (no noticeable corrosion). For a valid VIA test, the control sample must have grade 0 (heavy corrosion). The control samples consistently rated a grade 0 for all VIA tests; therefore, the test was valid. RH and the temperature of each test jar were monitored by sensors and data logging software. Samples subjected to the exhaustion test paper were inspected to ensure none had any surface pits that exceeded 300 mm in diameter.
Results
The VIA corrosion grading per TM-208 of these samples are summarized in Table 1. These tests results create concerns over the feasibility of the exhaustion test criteria. Corrosion behavior of CS in the presence of VCI-coated packaging papers is shown in Figures 1 and 2.
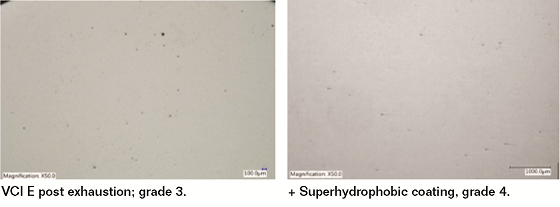
Papers coated with the superhydrophobic coating showed very promising results and coated samples passed the post-exhaustion VIA with gradings of 3 to 4. None of the superhydrophobic coated papers showed any surface pitting exceeding 300 µm after exhaustion VIA tests.
Two commercially available papers (VCI A and VCI B) failed the post exhaustion VIA tests, creating concerns about the exhaustion test criteria. One possible explanation for the failure is the exhaustion test conditions; 60 °C for 12 days while circulating air at 50% RH and a flow rate of 100 cm3/min can result in the loss of the VCI component on the wrap paper. Therefore, following exhaustion tests, the VCI quantity is not sufficient to protect the steel sample during the VIA tests. The other issue is the mechanism of VCI surface adsorption. When the VCI adsorbed onto the steel surface, its hydrophobic nature does not allow wetting of the surface that results in better corrosion protection. The exhaustion test on the wrapping materials removes (depletes) the protective VCI compounds and results in unacceptable VIA grading.
It is a more realistic approach to conduct the exhaustion tests on the wrapped steel samples with VCI packaging materials and subject those samples to the VIA tests. These tests demonstrate whether the VCI-adsorbed compound can maintain its attachment to the metal surface during the exhaustion tests, protecting steel samples against corrosion. By modifying the exhaustion tests to include the steel samples in the test chamber (wrapping steel samples with the VCI impregnated papers during the exhaustion cycle), VIA tests showed a very satisfactory performance for VCI A, VCI B, VCI C, VCI D, and VCI E, with a grading of 3 to 4. This modification in the exhaustion cycle allowed the VCI molecule to be absorbed on the steel surfaces; improved corrosion performance was seen for these VIA tests.
Conclusions
In summary, VCI-impregnated papers showed satisfactory corrosion protection in the VIA test method after exhaustion tests and steel samples achieved grade 4. Applying superhydrophobic coating showed a satisfactory performance for all impregnated papers. Two commercially available papers failed the post exhaustion VIA tests. This created concern over the feasibility of the exhaustion test criteria.
Conducting the exhaustion tests on the wrapped steel samples with VCI packaging materials and subjecting those samples to the VIA tests demonstrated satisfactory results of grade 3 to 4. Therefore, the MILSTD 3010C VIA and exhaustion test method might need to be re-evaluated and the exhaustion cycle procedure modified.
References and About the Authors