Deformed steel reinforcement (rebar) is a widely used steel product, especially in the construction industry, used in buildings, bridges, roads, etc.1 However, in the transportation and the storage process, uncoated rebar is often corroded by the corrosive environments. The corrosion product of the rebar surface reduces gripping force to concrete and affects the quality of the whole construction.
The rust on the rebar surface is one of the major factors that influences the durability of the building.2-3 As an anticorrosive measure for rebar, the use of oil has obvious drawbacks, such as high cost, environmental consequences, easy inflammability, and complicated post-treatment technology to restore proper rebar-concrete bonding.4
At present, there are obvious disadvantages in water-based conversion coatings. Some water-based coatings contain highly toxic substances, such as chromate or nitrite, some contain large amounts of phosphate, which causes severe environmental pollution due to the eutrophication, some contain lots of molybdate or noble metal and its inhibiting effect is not ideal. Other water-based antirust agents contain resin that forms a thick film on a metal surface, thus adversely affecting the bond between the rebar and concrete.5-7
With the increasing requirements on environmental protection and protective quality requirements, the traditional transit coatings cannot meet construction demands. Therefore, development of an environmentally friendly coating for bare rebar is very important.
Experimental Procedures
This study developed and investigated an environmentally friendly corrosion inhibitor for rebar. In this work, the composition of the conversion coating solution included: trisodium phosphate (Na3PO4) 10 g·L-1,sodium silicate (Na2SiO3) 200 g·L-1,diethanolamine [HN(CH₂CH₂OH)₂] 60 g·L-1,sodium gluconate (NaC₆H₁₁O₇) 60 g·L-1, alcohol ethoxylate [RO-(CH2CH2O)n-H R=C16-18, n=10-30] 1 g·L-1, 1,4 butanediol (HOCH₂CH₂CH₂CH₂OH) 100 g·L-1, potassium sodium tartrate (KNaC4H4O6·4H2O) 100 g·L-1, EDTA (C10H16N2O8) 40 g·L-1, sodium tetraborate [Na₂[B₄O₅(OH)₄]·8H₂O] 10 g·L-1, waterborne acrylic emulsion (solid content 42%) 40 g·L-1, and film-forming additive 4 g·L-1.
All the reagents were purchased from Sinopharm† and used as-received without further purification. Coating solution aliquots (1,000 mL) were prepared as follows: trisodium phosphate and sodium silicate were dissolved in 600 mL deionized (DI) water at 60 ℃. Under constant stirring, diethanolamine, sodium gluconate, alcohol ethoxylate, 1,4 butanediol, potassium sodium tartrate, EDTA, sodium tetraborate, waterborne acrylic emulsion, and the film-forming additive were slowly added to the solution in that order.
Then, the mixture was stirred until it became clear, and DI water was added until the total volume of the solution was 1,000 mL. Finally, the mixed solution pH was adjusted to 10 with 0.1mol·L-1 sodium hydroxide (NaOH). In this work, a water-based coating solution at a typical concentration of 30% of the mixture solution was then used to treat the specimens.
The rebar specimens used contained 0.25% C, 1.6% Mn, 0.8% Si, 0.045% S, 0.045 P, and the remainder Fe. The samples were degreased with soapy water, DI water, and final degreasing with acetone in an ultrasonic bath for 3 min. Then, the specimens were heated at 130 to 180 ℃ for 10 min, simulating hot rolling treatment process of the rebar, and then submerged in the conversion coating solution at 30 to 60 ℃ for 5 min. After air drying at room temperature for 24 h, the corrosion resistance of untreated and treated rebar was evaluated by electrochemical tests in a 3.5% aqueous sodium chloride (NaCl) solution, a salt spray test (SST), and a salt immersion test. The electrochemical tests were carried out in a 300 mL cell with a VersaSTAT 3† potentiostat-galvanostat.
The specimens were immobilized in the cell in a holder that exposed 100 mm2 of the specimen surface to the electrolyte (3.5% NaCl solution). A standard three electrode system was used that consisted of a working electrode (treated or untreated samples), counter electrode (platinum plate, 15 by 20 mm), and a saturated calomel reference electrode (SCE). All potentials were referenced to the SCE. All the specimens were immersed in the electrolyte for 30 min before each electrochemical test, which allowed the system to stabilize. The open circuit potential (OCP) values of the samples were monitored in 3.5% NaCl solution for 600 s. The potentiodynamic curves were generated by sweeping the potential from -350 to 350 mV vs. SCE (vs. OCP) at a scan rate of 1 mV/s. The tests were typically repeated three times to ensure reproducibility.
Corrosion resistance of the protective film was evaluated by a neutral SST performed according to ASTM B117.8 Samples were placed at a 45-degree angle in corrosion-resistant self-supporting polyethylene casing, inside a test chamber where a salt solution (5% NaCl with a pH of ~7) at 35 ℃ was sprayed. Corrosion resistance of specimens were monitored every 2 h until 24 h. The performance of the treated rebars were also evaluated through a salt immersion test. Treated and untreated rebars were immersed in a 5% NaCl solution at 25 ℃, and the corrosion conditions of the rebars under stationary state were recorded.
Results and Discussion
OCP
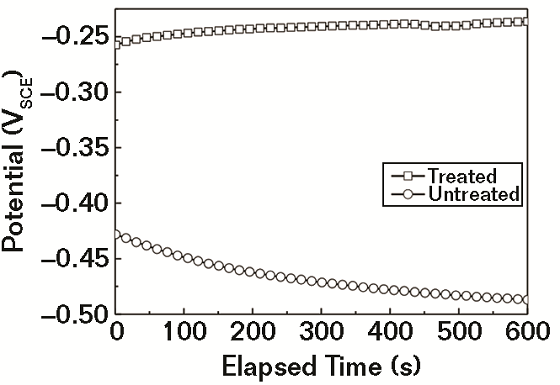
Figure 1 shows the variation of Eopc for treated and untreated specimens in 3.5% NaCl solution. During the early stages of immersion, the Eopc value of treated specimens increased slowly, while that of untreated specimens decreased slowly. The steady state for the tests was achieved in about 10 min, and the Eopc for treated specimen was clearly higher than that of the untreated specimen throughout the process, which indicates that the treated rebar has a lower corrosion potential than bare metal thermodynamically.9
Polarization Curves
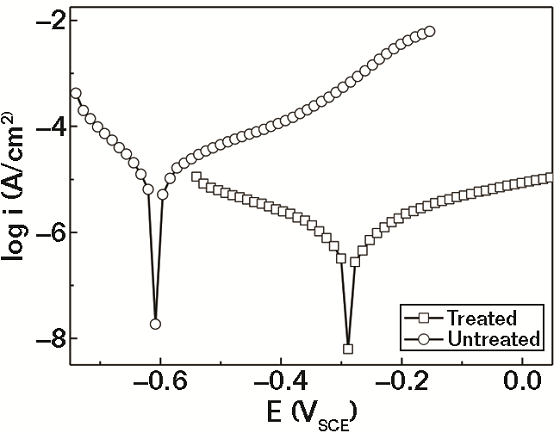
The polarization curves for typical test and control specimens in 3.5% NaCl solution are shown in Figure 2. The electrochemical parameters, corrosion potential (Ecorr), and corrosion current density (Icorr) were obtained and are shown in Table 1. The Ecorr of the treated rebar is considerably more positive (0.31 V) than that of the untreated specimen. Thermodynamically, the more electropositive the Ecorr, the less likely the material will corrode. So, the rebar that was treated with the conversion coating solution is more corrosion resistant than the untreated.10, 11

Generally, the cathodic polarization curves are assumed to represent the hydrogen evolution, while the anodic polarization curves denote the dissolution of the specimen. As shown in Figure 1, both anodic and cathodic CDs of the treated specimen have been significantly reduced when compared to the untreated rebar. The corrosion CD Icorr (Table 1) for the treated specimen is ~1/29 of that for the untreated specimen. This indicates that the corrosion rate of the rebar in 3.5% NaCl solution was greatly reduced after treatment and the conversion film can effectively protect the rebar from corroding.12
SST
An SST has the advantages of straightforwardness and accuracy and is often performed to evaluate corrosion protection of films and coatings on a metal surface.5 Figure 3 (left) shows an optical photograph of rebar that was partly treated. The treated surface has a homogeneous and smooth conversion film, which shows metallic luster. Figure 3 (right) shows the surfaces of the partly treated specimen after 12 h SST. The bare rebar had corroded in 2 h, after 12 h SST, it was corroded severely, and the surface was covered with a layer of rust as shown in the graph. The metallic luster remained on the treated portion and was almost not corroded. The SST result shows that the conversion film can provide effective protection for the rebar, which was consistent with the results of electrochemical testing.

Immersion Test in NaCl Solution
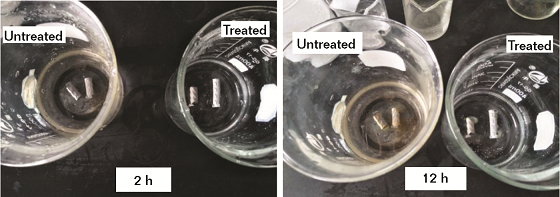
The immersion test in NaCl solution can reflect the static corrosion situation of metal in direct contact with water.13 The corrosion situation of the untreated and treated rebars immersed in 5% NaCl solution for 2 h and 12 h are shown in Figure 4. In 2 h, as shown in Figure 4 (left), the surface of untreated rebar and its surrounding solution appeared very light yellow in color, which was the color of iron ion, while the treated metal kept its metallic luster. This shows that the untreated tread steel had been corroded after 2 h of immersion in the 5% NaCl solution.
Figure 4 illustrates that the corrosion states for the treated vs. untreated rebar are vastly different after 12 h of immersion. In this case, the surface of bare tread steel was almost covered with a layer of yellow rust and the color of the surrounding solution became deeper. The treated metal showed no visible signs of corrosion on the surface and the surrounding solution of treated tread steel remained clear and colorless after 12 h of immersion. This indicates that the water-based film provided good corrosion protection for the substrate, which is consistent with the result of potentiodynamic polarization testing and SST.
Conclusions
In summary, this article presents an environmentally friendly coating, which can form homogeneous and smooth conversion film on rebar’s surface. The electrochemical test results show that, compared with untreated specimens, the treated specimens’ corrosion potential increased and corrosion current density decreased. The neutral SST results show that the treated rebar still retained a metallic luster with almost no corrosion after 12 h SST. The immersion test in the 5% NaCl solution demonstrated that the treated metal surface had no corrosion after 12 h of immersion. The conversion film can provide effective protection for the rebar.
Acknowledgement
The authors thank the financial support of the Scientific Research Fund of Provincial Natural Science Foundation of Hunan (2018JJ4046).
† Trade name.
References
1. T. Pham, M.N.S. Hadi, “Predicting Stress and Strain of FRP Confined Rectangular/Square Columns Using Artificial Neural Networks,” Journal of Composites for Construction 64 (2014): p. 279.
2. H. Guo, et al., “Corrosion mechanism and protection of reinforced concrete bridges,” Engineering Construction 38, 6 (2006): p. 13.
3. H. Geng, et al., “Optimization of anti-corrosion project plan for a bridge pier,” Henan Building Materials 1 (2012): p. 29.
4. Z. Tao, et al., “Adsorption Properties and Inhibition of Mild Steel Corrosion in 0.5 M H2SO4 Solution by Some Triazol Compound,” Journal of Materials Engineering and Performance 22 (2013): pp.774-781.
5. F. Wu, et al., “Silane-Zirconium Pretreatment for Mild Steel,” MP 57, 4 (2018): pp. 48-51.
6. K. Kowalczyk, “Zinc-free varnishes and zinc-rich paints modified with ionic liquids,” Corros. Sci. 78 (2014): pp. 111-120.
7. C. An, “Study on Rust Remover and Antirust Fluid for Steel Pieces of Process,” Surf. Technol. 31, 2 (2002): p. 40.
8. ASTM B117-16, “Standard Practice for Operating Salt Spray (Fog) Apparatus” (West Conshohocken, PA: ASTM International, 2016).
9. F. Wu, et al., “The Effect of Combined Corrosion Inhibitors on AZ31 Magnesium Alloy,” MP 55, 5 (2016): p. 42.
10. Z. Tao, et al., “Tetraoxa-Diphosphaspiro Derivative as Suppressor for Microvia Filling by Copper Electroplating in Acidic Solution Electrochemical/Electroless Deposition,” J. Electrochem. Soc. 164, 14 (2017): p. 1,034.
11. F. Wu, S. Zhang, Z. Tao, “Corrosion behavior of 3C magnesium alloys in simulated sweat solution,” Mater. Corros. 62, 3 (2011): p. 234.
12. Z. Tao, et al., “Synergistic Effect of Different Additives on Microvia Filling in an Acidic Copper Plating Solution Electrochemical,” J. Electrochem. Soc. 163, 8 (2016): p. 379.
13. F. Wu, X. Liu, X. Xiao, “Corrosion resistance and characterization studies of calcium series chemical conversion film via green pretreatment on magnesium alloy,” Anti-Corrosion Methods and Materials 63, 6 (2016): pp. 508-512.