Carbon capture and storage (CCS) technology is now considered to be one of the most effective options for reducing the carbon dioxide (CO2) level in the environment. The CCS process involves three stages: capture of CO2 from the large point sources, such as heat power stations and refineries; compressing it into a supercritical or liquid state and transporting it to the storage sites; and injecting it into the sequestration reservoirs, including depleted oil and gas reservoirs and underground aquifers.1
It is known that dry CO2 is not corrosive to carbon steels (CS).2 However, to reduce both energy and capital costs, in general, the CO2 to be sequestered normally contains water (H2O), which causes the steels used for CO2 transportation to suffer CO2 corrosion, and consequently damage the integrity of steels.3 The corrosion behaviors of steels under supercritical CO2 (SC CO2)/ H2O systems have been investigated by many researchers.1-5 In addition to H2O, other impurities may also be included in the CO2 streams, due to their different sources, and include SOx, NOx, oxygen (O2), carbon monixide (CO), methane (CH4), and hydrogen sulfide (H2S). Some researchers have investigated the effect of sulfur dioxide (SO2) on the corrosion behavior of CS under SC CO2 conditions, while fewer studies were focused on the effect of impurities H2S, CO, or O2, which also significantly affect the corrosion of steels under SC CO2 conditions.6-7 In addition, investigation of the corrosion behaviors of Cr-containing steels is limited.
Therefore, in this study, the effects of O2, CO, and H2S contents on the CS and Cr-containing steel in SC CO2 conditions were studied.
Experiment
The test materials in this study included one CS (API 5L X65) and one Crcontaining steel (13 Cr steel). The corrosio
n experiments were performed in a high-temperature and high-pressure autoclave. The impurities in this study were O2, CO, and H2S and their contents were 3 to 5 ppm (low level), 300 to 400 ppm (high level), and 23,000 to 25,000 ppm (super high level). The duration for all the corrosion experiments in this study was 96 h at 80 °C and 13.5 MPa.
After exposure, the samples were pickled in 10% hydrochloric acid (HCl) inhibited with 10 g/L hexamethylenetetramine, then rinsed sequentially in deionized water, acetone, and dried with hot air. Then, the corrosion rate of steel under different conditions was calculated by the weight loss method.4 Table 1 shows the observed corrosion rates.
Results and Discussion
Effect of O2
The corrosion rates of CS and Cr- containing steel exposed in SC CO2 saturated water with different O2 contents for 96 h are shown in Figure 1. It was obvious that with increasing O2 content, the corrosion rate of CS increased first and then decreased. When O2 content was low, the corrosion rate of CS increased slowly, while it increased rapidly in the presence of a high level of O2 content. However, when the O2 content continued to increase into a super high level, the corrosion rate of CS significantly decreased to a lower rate than that without O2 exposure.
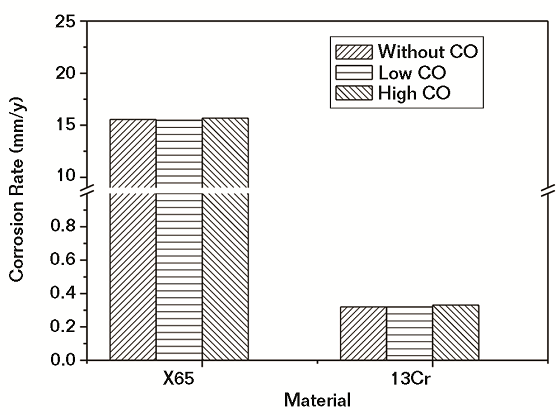
Figure 1 shows that the corrosion rate of Cr-containing steel decreased with an increase of O2 content. When the O2 content increased from zero, low level to high level, the corrosion rate of Cr-containing steel gradually decreased. However, when the O2 content increased to a super high level, the corrosion rate of Cr-containing steel rapidly decreased to only 0.06 mm/y.
It is known that the corrosion rate of steel in a CO2 environment is mainly determined by the cathodic reaction rate. The rate of cathodic reaction, in the presence of O2, is much higher than that of CO2 exposure,8-9 so when the concentration is equal, the corrosion of O2 to steel is ~20 times as much as that of solely CO2 exposure.9 When CO2 and O2 coexist, the corrosion rate of steel will be higher than that of pure CO2. Therefore, even a low-level content of O2 can significantly accelerate the corrosion process of CS in a predominately CO2 environment.
When the concentration of O2 is very high, the driving force of the cathode reaction increases significantly. As the rate of cathode reaction increases, the concentration of OH– increases, resulting in an increase of solution pH. Studies from Nesic, et al.,3,10 showed that an increase in pH will promote the deposition of a protective corrosion film product on a steel surface, thereby reducing the corrosion rate. At the super high oxygen concentration, O2 not only does not accelerate the corrosion of the steel, but also exhibits a passivating effect, which is like that observed in other studies.9 The corrosion rate of Cr-containing steel decreased with increases in O2 content, which is due to the effect of the Cr element on the corrosion resistance of steel.11 On one hand, the addition of the Cr element increases the electrode potential of the steel, thus improving the ability of steel to resist electrochemical corrosion. On the other hand, the addition of the Cr element can make the surface of the steel form stable passivating film, such as chromium hydroxide [Cr(OH)3], chromium oxide (Cr2O3), and other Cr-compounds, which are firmly bonded to the steel matrix.
Effect of CO
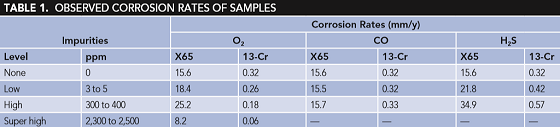
Figure 2 shows the corrosion rates of CS and Cr-containing steel exposed in water saturated with SC CO2 with different CO contents for 96 h. It could be found that even a high-level content of CO showed nearly no effect on corrosion rates of both CS and Cr-containing steel. This is because CO does not participate in the corrosion reactions, therefore it shows no effect on steel corrosion. The change in the corrosion rate of Cr-containing steel in the presence of different contents of CO was <0.01 mm/y, which may come from measurement error.
Effect of H2S
The corrosion rates of CS and Cr-containing steel exposed in water saturated with SC CO2 with different H2S contents for 96 h are shown in Figure 3. It was obvious that with increasing H2S content, the corrosion rates of both CS and Cr-containing steel increased. The corrosion rate of CS increased slowly when H2S content increased from none to a low-level content of H2S. However, when H2S content increased to a high level, the corrosion rate of CS rapidly increased to a level that was more than two times the corrosion rate in the absence of H2S. The corrosion rate of Cr-containing steel could be increased by ~30%, even in the presence of low-level H2S content. As the H2S content continued to increase to a high level, the corrosion rate of Cr-containing steel continued to increase to levels much higher than the corrosion rate under H2S-free conditions.
Among CO2, O2, and H2S, the solubility of H2S in water is highest. In water with a H2S/CO2 system, once H2S is dissolved in water, it is immediately ionized, which makes the water become acid and causes corrosion damage to the metal.12-13 HS– and S2– could be adsorbed on the surface of the metal, forming the adsorbed complex ion, Fe(HS)–, which makes the metal potential shift to negative value and causes the acceleration of the cathodic hydrogen release. Thus, H2S not only acts as a catalyst for the anode process and promotes the dissolution of iron, but also provides S2– for the formation of the corrosion product film, FeS, on the steel surface.
Besides FeS, other sulfide compounds are formed in the corrosion product film, which can have a variety of crystal structures, such as mackinawite (FeS1−x), pyrrhotite (Fe7S8), troilite (Fe1−xS), pyrite (FeS2), and greigite (Fe3S4).3,14-15 On one hand, these sulfide compounds covered on the steel surface were usually not dense and could not prevent the ion from reaching the steel surface from the solution. At the same time, a galvanic couple could be formed between sulfide compounds and the steel surface, causing the steel surface to continue to be corroded, often accompanied by pitting. On the other hand, as the solution is acid in the presence of H2S, the protective corrosion product film ferrous carbonate (FeCO3) is difficult to form. That is why the corrosion rates of CS and Cr-containing steel in the H2S/CO2 system were higher than that of other conditions.
Comparison of the Effect of O2, CO, and H2S
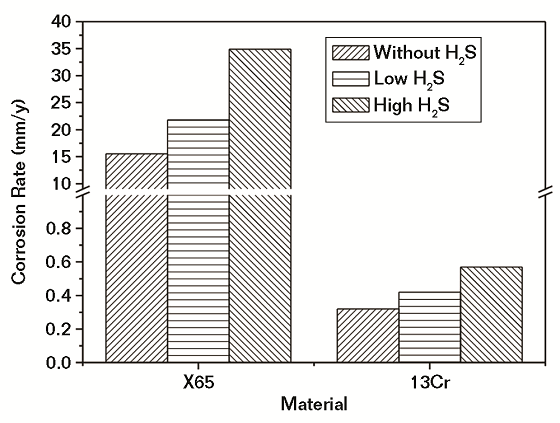
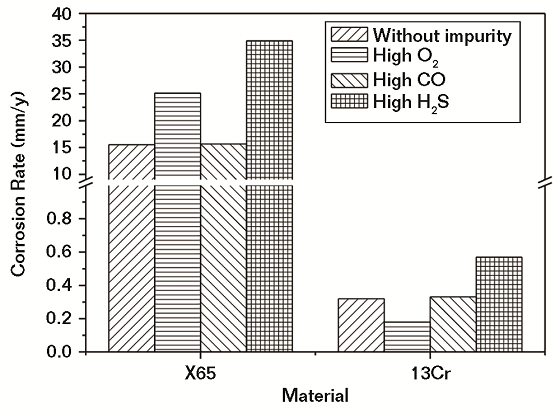
The corrosion rates of CS and Cr-containing steel in water saturated with SC CO2 with and without different contents of impurities after 96 h of exposure are shown in Figures 4 and 5, respectively. It was obvious from Figure 4 that the corrosion rates of both CS and Cr-containing steel were nearly the same with and without low-level CO content. In the presence of low-level O2 and H2S content, the corrosion of CS increased, and it was higher in the H2S environment. For Cr-containing steel, the corrosion rate decreased in the presence of O2 and increased under the H2S environment.
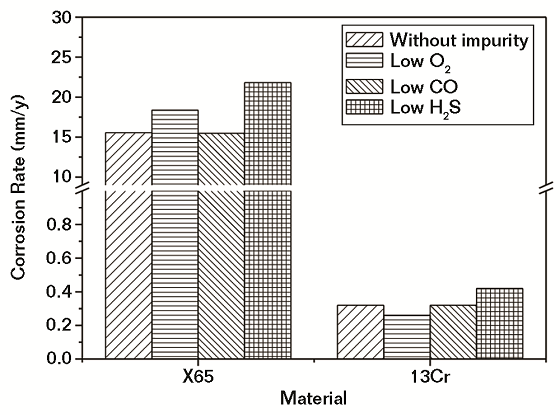
In Figure 5, note that the variations of corrosion rates for both CS and Cr-containing steel with high-level content of impurities followed the same pattern as that under conditions with a low-level content of impurities. However, a high-level content of H2S was more effective at increasing the corrosion rate of both CS and Cr-containing steel than a low-level content of H2S, while a high-level content of O2 was more effective at increasing the corrosion rate of CS and decreasing the corrosion rate of Cr-containing steel than a low-level content of O2. As a whole, the effect of different impurities on the corrosion rate of steels increased from CO, O2, to H2S. H2S showed maximum effect while CO had the smallest effect. The higher the content of impurities, the greater effect it showed.
Conclusions
The corrosion rates of CS and Cr-containing steel in water saturated with supercritical CO2 with different contents of impurities were investigated. The following conclusions were drawn:
1. CO showed nearly no effect on the corrosion rate of both CS and Cr- containing steel in all cases.
2. As the content of impurities increased, the corrosion rate of CS increased in both O2 and H2S environments, while it decreased in the presence of O2, and increased under H2S conditions for Cr-containing steel.
3. With the same content level of impurities, the corrosion rates of CS and Cr-containing steel were higher in the H2S environment than under O2 exposure.
References
1 Y.C. Zhang, et al., “The Relationship Between Fracture Toughness of CO2 Corrosion Scale And Corrosion Rate of X65 Pipeline Steel Under Supercritical CO2 Condition,” International J. of Greenhouse Gas Control 5, 6 (2011): pp. 1,643-1,650.
2 Y.C. Zhang, K. Gao, G. Schmitt, “Effect of Water on Steel Corrosion Under Supercritical CO2 Conditions,” MP 50, 6 (2011): pp. 62-68.
3 S. Nesic, “Key Issues Related to Modelling of Internal Corrosion of Oil and Gas Pipelines— A Review,” Corros. Sci. 49, 12 (2007): pp. 4,3084,338.
4 Y.C. Zhang, et al., “Discussion of the CO2 Corrosion Mechanism Between Low Partial Pressure and Supercritical Condition,” Corros. Sci. 59 (2012): pp. 186-197.
5 Y.S. Choi, et al., “Wellbore Integrity and Corrosion of Carbon Steel in CO2 Geologic Storage Environments: A Literature Review,” International J. of Greenhouse Gas Control 16, Supplement 1 (2013): pp. S70-S77.
6 Y.S. Choi, S. Nesic, D. Young, “Effect of Impurities on the Corrosion Behavior of CO2 Transmission Pipeline Steel in Supercritical CO2-Water Environments,” Environmental Science and Technology 44, 23 (2010): pp. 9,233-9,238.
7 Y.S. Choi, et al., “Effect of H2S on the Corrosion Behavior of Pipeline Steels in Supercritical and Liquid CO2 Environments,” CORROSION 2015, paper no. 5927 (Houston, TX: NACE International, 2015).
8 G.T. Skaperdas, H.H. Uhlig, “Corrosion of Steel by Dissolved Carbon Dioxide and Oxygen,” Industrial & Engineering Chemistry Research 34, 6 (1942): pp. 748-754.
9 T.J. Finnegan, et al., “Corrosion of Steel— Quantitative Effect of Dissolved Oxygen and Carbon Dioxide,” Industrial and Engineering Chemistry Research 27 (1935): pp. 774-780.
10 S. Nesic, et al., “A mechanistic Model for Carbon Dioxide Corrosion of Mild Steel in the Presence of Protective Iron Carbonate Films—Part 2: A Numerical Experiment,” Corros. Sci. 59, 6 (2003): pp. 489-497.
11 H. Inaba, M. Kimura, H. Yokokawa, “An Analysis of the Corrosion Resistance of Low Chromium-Steel in a Wet CO2 Environment by the Use of an Electrochemical Potential Diagram,” Corros. Sci. 38, 9 (1996): pp. 1,4491,461.
12 D. Rickard, “Kinetics of FeS Precipitation: Part 1. Competing Reaction Mechanisms,” Geochim. Cosmochim. Acta 59, 29 (1995): pp. 4,367-4,379.
13 L. Zhang, et al., “Effect of H2S Concentration on the Corrosion Behavior of Pipeline Steel Under the Coexistence of H2S and CO2,” International J. of Minerals, Metallurgy, and Materials 21, 4 (2014): pp. 388-394.
14 A. Davoodi, et al., “A Comparative H2S Corrosion Study of 304L and 316L Stainless Steels in Acidic Media,” Corros. Sci. 53, 1 (2011): pp. 399-408.
15 J.W. Tang, et al., “The Effect of H2S Concentration on the Corrosion Behavior of Carbon Steel at 90 °C,” Corros. Sci. 52, 6 (2010): pp. 2,050-2,058.