Carbon dioxide (CO2) capture and sequestration (CCS)
technologies can play an important role in decreasing greenhouse gas emissions
by greatly reducing the amount of CO2 released from new and existing
coal- and gas-fired power plants as well as large industrial sources such as
cement production and natural gas processing facilities.1 The U.S. Inventory of Greenhouse Gas Emissions
and Sinks estimates that more than 40% of CO2 emissions in the
United States is a result of electric power generation;2 and
according to the International Risk Governance Council, electric power
plants are responsible for approximately one third of global CO2
emissions.3
Burning fossil fuels (e.g., coal)
to produce electricity emits flue gases that contain CO2, water vapor, sulfur
oxides (SOx), and nitrogen oxides (NOx). There are several methods currently utilized
to capture CO2 from flue gases. The post-combustion method,
which can reduce a power plant's carbon emissions by 80 to 90%, is well
understood and widely used in the natural gas industry. This method uses a reactive absorption process with amine
solvents to separate and capture the CO2 from the flue gases
after the fossil fuel is burned, explains NACE International member Sridhar Srinivasan, global business
leader—Corrosion Center of Excellence with Honeywell International, Inc.
(Houston, Texas) and chair of NACE’s Technology Exchange Group (TEG) 100X,
Sensors: Corrosion and Corrosiveness Sensor Technology, and vice chair of
Specific Technology Group (STG) 62, Corrosion Monitoring and Measurement—Science
and Engineering Applications. The CO2 is captured by passing the flue gases through the liquid
amine solvent and the amines
selectively dissolve and absorb the CO2 gas. The solvent can be
regenerated by heating, which releases the water vapor and leaves a
concentrated stream of CO2 that can be transported
to storage. Different types of
amines are typically used (e.g., monoethanolamine [MEA], methyldiethanolamine
[MDEA], and others depending on the type of
application and operating conditions).
According to Srinivasan, CO2 gas is
extremely corrosive when it comes into contact with water; and once the amines
are loaded with the absorbed CO2 gas, they can be very corrosive to
the metallurgy of the CO2 capture plant due to their high oxygen and
CO2 levels as well as impurities such as fly ash, SOx, and NOx. Corrosion mechanisms seen in CO2
capture plants include general corrosion, stress corrosion cracking (SCC),
crevice corrosion, pitting corrosion, and erosion-corrosion. Additionally,
corrosion can be exacerbated by oxidative and thermal degradation of the amine
solvent, which can vary with a CO2 capture plant’s run time and gas
throughput.

“Corrosion is a profound safety issue in industry,” says Srinivasan.
“Most plant accidents happen because there is either inadequate or ineffective
corrosion management.” He notes that corrosion is a dynamic phenomenon—it
changes in real time as the process changes. “When you look at corrosion, it is
similar to looking at the changes in blood pressure of a person who is
undergoing different activities. The changes in corrosion are a function of a
number of process variables [feed rates, temperature excursions, variations in
product purity, etc.].” Because of that, he adds, process plants have to be
extremely careful when managing and mitigating corrosion, especially with acid
gases like CO2.
The way to accurately and meaningfully track corrosion is with online
corrosion monitoring in real time, Srinivasan says. For decades, however, process
corrosion has been monitored with manual, offline techniques that indicate the
presence of corrosion after it has occurred in the process equipment. This, he
notes, leads to the accumulation of corrosion that can cause failures and
outages, and corrosion management that is reactive rather than proactive. While
common corrosion monitoring techniques, such as linear polarization resistance
(LPR) probes, can be used in online monitoring systems, typically the protocols
to link corrosion data to a plant’s process control systems have not been sufficient.
Now, due to advanced plant process control system technology that is
being used to regularly monitor plant process conditions, it is possible to
incorporate a relatively new corrosion monitoring approach. Industrial plants have
a distributed control system (DCS) where process data are sent and evaluated
for production optimization. Online corrosion monitoring technology can also send
real-time corrosion data to the plant’s DCS where they can be regularly
monitored along with process control data, making it possible to correlate
changes in corrosion rates with process events. This proactive, online approach
allows plant operators to determine the presence and cause of corrosion and
make process changes before substantial corrosion damage occurs.
Real-Time, Online Monitoring Technology
The online corrosion monitoring technology
measures the minuscule electrical current that results from corrosion occurring
in the system. The technology utilizes a sensor device that comprises a
three-electrode corrosion probe and a transmitter. The three electrodes are
identical and function as the working electrode, reference electrode, and
auxiliary/counter electrode (which supplies current to the working electrode).
Since they are constructed of the same metallurgy as the piping material being
monitored, the electrodes are essentially replicating the corrosion behavior of
the pipe, says Srinivasan. The transmitter measures and analyzes the current
flow and sends it to the DCS.
By integrating measurements from multiple electrochemical monitoring
techniques—LPR, harmonic distortion analysis (HDA), and electrochemical noise
(ECN)— the technology is able to characterize the corrosion rate and
pitting factor every 30 seconds, and provide four output variables to
accurately quantify corrosion that stems from oxidation of the metal. The LPR technique, Srinivasan explains,
defines the polarization resistance by applying a potential to the working
electrode and measuring the current flow between the working electrode and the
counter electrode. By finding the polarization resistance, which is inversely proportional to the measured corrosion current,
he adds, the technique can determine the
overall rate of metal loss (corrosion rate).
Because each corroding system has its own
unique behaviors (signatures), the HDA technique is used to determine
variations in the current, known as the harmonics, to find the localized Stern
Geary constant (B value). The B value represents a system’s “constant,” which
is determined by the mechanism and kinetics of the system’s corrosion process.
The B value can be different for each corroding system depending on the
characteristics of the system and the type of corrosion activity (i.e., the
anodic and cathodic currents and potentials, which can vary quite a bit from
system to system). However, the LPR technique typically uses a default B value
to calculate the corrosion rate, which can lead to significant
errors when computing the corrosion rate. By using a measured B value, a more
accurate corrosion rate can be determined.
The corrosion process also generates ECN,
which is the low-amplitude (<1 mV), low-frequency fluctuation of corrosion
current and potential. The ECN technique measures these low-level fluctuations
between working and counter electrodes, which can determine the type and speed
of corrosion. Based on the magnitude of the variations in the corrosion current
(intrinsic current noise), Srinivasan comments, the ECN measurement can
establish whether the corrosion is likely to be uniform or localized and be
used to determine the pitting factor, which indicates the probability of the
corrosion mechanism generating localized corrosion over time. Typically,
general corrosion processes have low levels of ECN, but the onset of localized
corrosion (e.g., pitting) leads to increasingly higher levels of ECN.
The last output delivered by the
electrochemical monitoring techniques is the corrosion mechanism indicator
(CMI), which shows the presence and likely effects of surface films. This is
determined by the characteristics of the interface between the metal and the
environment (electrolyte) and its influence on the behavior of the corrosion
current.
The four output variables from the monitoring
device are calculated continuously at time intervals determined by the plant
operator, and immediately communicated to the plant DCS via hardwire or
wireless channels, or stored remotely for later download. Since the reported
corrosion data represent real-time measurements, Srinivasan notes, the plant
operators are able to correlate corrosion events with specific process
changes (temperature, flow rate, injection of neutralizers or catalysts, etc.)
and determine potential cause-effect relationships.
Corrosion Monitoring in a CCS Pilot Plant
To investigate the corrosion process at the
post-combustion CCS pilot plant at the Maasvlakte coal-fired
power plant in Rotterdam, The Netherlands, the advanced, real-time online
corrosion monitoring technology was implemented in the unit’s CO2
capture process. The objective of the study was to verify the interrelationship
between amine solvent degradation, ammonia (NH3) emissions from
oxidative solvent degradation, and corrosion, as well as validate the accuracy
of the online corrosion monitoring system in terms of reporting the correct
corrosion rate. The study was conducted over two monitoring campaigns during a
two-year period, with each campaign running about five to six months. Corrosion
coupons were also installed parallel to the online corrosion monitoring device
to provide baseline corrosion data for validating results from the online
corrosion monitoring system. A review of the monitoring approach and results of the
case study were presented during the CORROSION 2015 symposium, “Corrosion
Monitoring Technologies: Past Present and the Future,” sponsored by TEG 100X.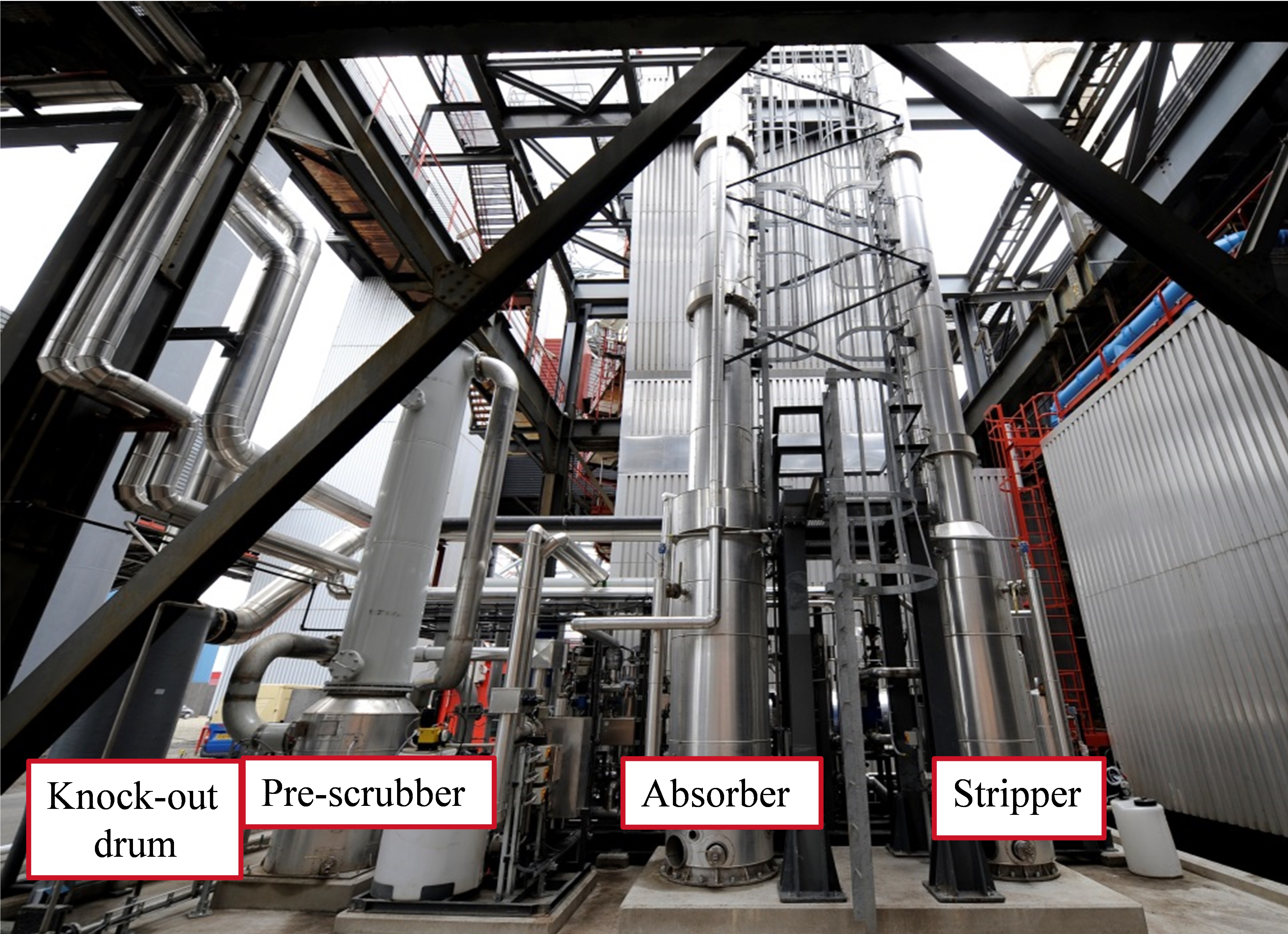
The CCS pilot plant components are mainly
comprised of 304L (UNS S30403) and 316L (UNS S31603) austenitic stainless steel
(SS) to minimize general corrosion. For both monitoring campaigns, 30 wt% MEA
was used as the CO2 capture solvent. Based on an evaluation of the
plant’s CO2 capture process, the online corrosion monitoring device
was placed in an area suspected to be particularly susceptible to
corrosion—between the lean solvent pump and the amine stripper. In this portion
of the process, changes in amine composition, as well as degradation products
and impurities, operating temperature, local flow rates, and localized
turbulence corrosion, often affect the corrosion rate. “Corrosion behavior is a
function of the solvent concentration,” Srinivasan notes. “Depending on the
solvent, you will see different corrosion behaviors.”
The First Campaign
The first monitoring campaign ran for ~149 days and comprised ~2,200
operating hours. The campaign was divided into four periods. The same solvent
was used for the first 128 days, then completely replaced for the remainder of
the campaign. The flue gas contained 13 vol% CO2 and 7 vol% O2
for the first, third, and fourth operating periods, and 4 vol% CO2
and 17 vol% O2 during the second operating period. Online, real-time
corrosion monitoring was done during the third and fourth periods.
Two days after start-up during the third period (Day 110), the corrosion
rate peaked at ~800 µm/y. On Day 115, pure MEA was added to the unit as solvent
make-up, and the corrosion rate value decreased. With continued operation,
however, the corrosion rate increased to a peak of ~1,400 µm/y after several
weeks. On Day 128 the unit was cleaned and the solvent completely replaced, and
the corrosion rate decreased to <20 µm/y with only periodic low-level peaks
during the rest of the campaign.
The pitting factor values were also tracked during the campaign and
varied between 0.2 and 0.4, which is within the range for pitting (0.1 to 1.0).
The highest pitting factor values were recorded during periods when the general
corrosion rate measurements were low; and periods with high corrosion rates
showed very low pitting factor values. According to the presenters, these
measurements suggest that the corrosion was likely general in nature and not
related to pitting attack of the process unit. They also note that the peak
corrosion rate measured would be considered high for SS, but the time-averaged
corrosion rate values indicated a maximum metal thickness loss rate of 600
µm/y. While this corrosion rate would have produced a metal thickness loss of
only 0.03 mm during the 20 days of the campaign when corrosion rates were high,
the presenters point out that this corrosion rate would have resulted in a
thickness loss of 0.06 mm/y had the situation continued for a year, which is
considered excessive for SS in amine service.
The corrosion coupons placed in the hot lean solvent stream were lost in
the system during this campaign, and corrosion data from the online monitoring
process could not be validated.
The Second Campaign
The second monitoring campaign ran for ~140 days for a total of ~1,700
operating hours, and was divided into three periods. The CO2 capture
plant operated intermittently during the first period and continuously during
the second period; and then the solvent was completely replaced and the plant
ran continuously during the third period. The flue gas during all three periods
usually contained 13 vol% CO2 and 7 vol% O2. Corrosion
monitoring was performed over the entire duration of the campaign.
The corrosion rate measurements varied during the intermittent
operation, with peak corrosion rates ranging from 10 to 25 µm/y. During
continuous operation, the corrosion rates typically ranged between 2 to 5 µm/y,
but peaked at 30 µm/y prior to the solvent replacement on Day 65 and then
remained stable at ~5 µm/y. Based on the corrosion coupon inserted during this
campaign, the calculated corrosion rate was 0.3 µm/y, which was comparably low
to the much more sensitive online monitoring results. According to the
presenters, the consistently low corrosion rate values for both the online and
coupon readings are considered acceptable for SS in a passive condition in
amine service. Generally, the pitting factor values did not fall within the
range of values that indicate pitting, which suggests very low rates of general
corrosion although pitting factor readings did reach ~1.0 in transient periods
during intermittent operation.
The presenters report that a major corrosion effect observed in both
campaigns was an increase in the general corrosion rate over time during
continuous operation. The first campaign, however, showed higher corrosion
rates than the second. After a supplemental analysis of the amine solvent for
metal ions, they found that the metal ion concentration for the first
monitoring campaign was higher than it was for the second. The flue gas in the
first campaign also had higher oxygen content. They noted these conditions are
known to promote degradation of the amine solvent and formation of corrosive
reaction products. While in situ corrosion most likely contributed to the metal
ions being present in the amine solvent, the presence of metal ions promoted
oxidative degradation of the solvent, which resulted in conditions that further
degraded the solvent and led to increased corrosion in the unit. To reduce the
corrosion rate, the solvent needed to be completely replaced.
Srinivasan notes that the study demonstrated the efficacy and accuracy
of the real-time, online monitoring system and its value in immediately
identifying corrosive operating modes during plant operation, which will enable
plant operators to take remedial actions to restore acceptable corrosion
conditions on a real-time basis so corrosion damage can be avoided. “Corrosion
is hard to see, it happens over a period of time,” he says. “If we’re able to
monitor it in a reasonable time frame—real time—we’re going to be able to catch
it, fix it, or prevent it.”
More information on the real-time online monitoring technology and the
monitoring campaigns at the Maasvlakte coal-fired power plant can be found in
CORROSION 2015 paper no. 5954, “Plant Applications of Online
Corrosion Monitoring: CO2 Capture Amine Plant Case Study,” by R.D.
Kane, S. Srinivasan, P. Khakharia, E. Goetheer, and J. Mertens.
References
- “Carbon Dioxide Capture and Sequestration,” U.S. EPA, http://www.epa.gov/climatechange/ccs (April 13, 2015).
- “Draft Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990-2013,” National Greenhouse Gas Emissions Data, U.S. EPA, February 2015, http://www.epa.gov/climatechange/ghgemissions/usinventoryreport.html (April 13, 2015).
- “Power plant CO2 capture technologies,” International Risk Governance Council, http://www.irgc.org/issues/carbon-capture-and-storage/power-plant-co2-capture-technologies (April 13, 2015).