The most important factors affecting soil corrosivity considered in this study were electrical resistivity, pH, redox potential, sulfides, moisture, soluble salts, chlorides, and cations content. The corrosion rate of soil in different areas of the central desert of Iran is in the range of 0.029 to 0.097 mm/y and 0.037 to 0.108 mm/y with respect to field and laboratory corrosion experiments, respectively.
Soil corrosivity is a multidisciplinary challenge that has a huge effect on the structural integrity of metallic infrastructure. As a result, the study of the soil as a corrosive environment has become critically important.1
The most important cause of the destruction of buried pipes and tanks is soil corrosion.2-3 There are several million kilometers of pipelines around the world and most of them are buried in the soil. Koch et al.4 reported that the annual cost of corrosion in the drinking water and sewer system sectors in the United States is about US$36 billion. The costs associated with corrosion of pipes and tanks buried in the ground have been reported in many countries.
Therefore, to assess the risks and costs of failures, identifying the amount of soil corrosivity in any area is necessary and can lead to appropriate decisions to reduce corrosion. The variation in structure and chemical composition of the soil, even over a single site, can change the soil corrosion rate from one location compared to another location.
In this study, the corrosivity of soils in the different locations of central desert regions of Iran on St 37 steel have been investigated and several soil properties that are thought to contribute to soil corrosivity have been critically reviewed.
Materials and Methods
In this research, the corrosivity of the soils of the central desert of Iran was investigated. Regarding soil sampling, and depending on the extent of the area, four regions were considered as indicators. These regions with specific geographical locations were named as CR1, CR2, CR3, and CR4. In this study, a 10-point scoring method proposed by AWWA C1055 was used to evaluate soil corrosivity.
In this method, the most important soil properties such as soil electrical resistance, pH, moisture content, oxidation-reduction potential, and amount of sulfide are measured to determine soil corrosion potential. To determine the corrosivity potential of the soil, each of the listed parameters is given a score based on the coefficients in Table 1. The sum of the calculated points is considered as a criterion for determining the amount of soil corrosivity. If the total score for the five properties is greater than 10, the soil is considered as soil with high corrosion potential.
The corrosion rate of steel coupons was measured using weight loss and linear polarization methods. The coupons used for field and electrochemical corrosion experiments were selected from St 37 steel. For field weight loss tests according to NACE TM0169,6 10 cm × 10 cm × 0.5 cm coupons were used.
The surfaces were polished and were degreased and cleaned with acetone in an ultrasonic bath and distilled water. The samples were buried in the soil at a depth of 1.5 m and were removed from the burial sites after 45, 90, and 180 days, respectively. The corrosion products were removed from the surface and corrosion losses were immediately measured by an electronic balance with 10-4 gr precision.
For electrochemical corrosion tests, coupons with dimensions of 2 cm × 2 cm were prepared. In the electrochemical corrosion measurements, soil electrolyte was used because the soil has a very high electrical resistance and passes a very small electrical flow with the equipment used.
For preparation of the soil electrolyte, dry soil (dried at 120 °C for 4 h) and distilled water were mixed in a 1:2 ratio and then filtered through a No. 40 sieve after 12 h of mixing.
The electrochemical measurements were conducted in the filtered solution using a three electrode electrochemical cell, including working electrode, standard silver/silver chloride (Ag/AgCl) reference electrode, and a platinum electrode with an effective surface of 1 cm2 as the counter electrode.
Polarization tests according to ASTM G597 in soil electrolyte was performed by a frequency response analyzer-equipped potentiostat/galvanostat (Autolab Model 302†) device. Before polarization scanning, the working electrode was immersed in the test electrolyte for 24 h until a steady state potential was attained.
In this research, soil classification was performed using the 10-point method and
weighting properties. Then, field and electrochemical corrosion tests were performed on prepared steel samples. Finally, results of field and electrochemical experiments, 10 point, and weighting properties were compared with each other.
Results and Discussion
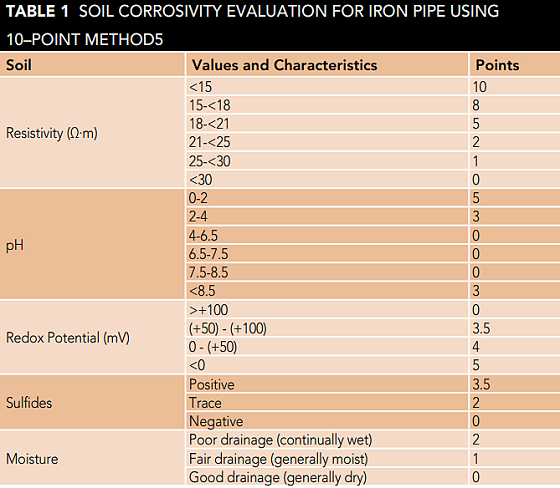
Table 2 shows the results of the electrical and chemical experiments performed on the soils in areas CR1, CR2, CR3, and CR4. The low electrical resistance of the soil facilitates the movement of electrons and thus accelerates the occurrence of the corrosion processes. The electrical resistivity of the soil in the CR4 region is the lowest and is therefore assigned the highest value (10 points) in the 10-point method. The electrical resistivity increases for the soils of CR3, CR2, and CR1 zones, respectively.
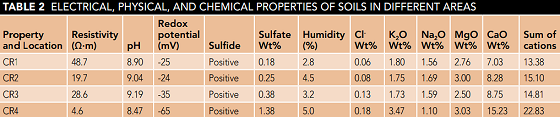
Electrical resistivity is influenced by a range of parameters such as dissolved salts, metallic cations, moisture, and texture. Dissolved salt ions and metallic cations function as charge carriers and giving rise to the current-carrying capability.8-10
As shown in the Table 2, the sum of the cations and chloride ion content for the soil of the CR4 zone is the highest. Another important factor affecting soil electrical resistivity is the amount of soil moisture. Increasing the moisture contents results in improved soil conductive linkage and consequently reduced soil electrical resistance.11-12
The decrease in the number of cations, chlorine ions, as well as moisture has led to a significant increase in the amount of electrical resistance to 48 Ω∙m for CR1 soil. However, a soil with a high and low pH indicates a high amount of dissolved salt in the soil, which increases the electrical conductivity of the soil. Therefore, the most suitable condition is soil with a neutral pH.
Results of pH measurements show that the soils in all four zones are in the pH range of 8.47 to 9.19 and in the low alkaline range, which are classified as non-corrosive soils without considering other corrosion factors. In the 10-point method, soil CR4 with pH equal to 8.47 was assigned a zero score and for the other three soils with pH greater than 8.5, a score of 3 was assigned.
The particles in clayey soils are more tightly packed and due to their high-density clays are very prone to anaerobic conditions and therefore have a low redox potential. On the other hand, higher density leads to poor aeration. Redox potential indicates whether a soil can maintain sulfate-reducing bacteria (SRB).13 Low redox potential indicates that soil oxygen content is low and therefore there are ideal conditions for SRB proliferation.
The sulfate-reducing bacterium is a specific group of microbes that uses sulfate as the electron capture polarizer for oxygen exchange and hydrogen sulfide (H2S is) produced as the end product. The widespread presence of these bacteria results in extensive corrosion in metal parts. Soil with redox potential above +100 mV have appropriate aerated conditions to delay bacterial growth.
In this study, the redox potential was determined to be -25, -24, -35, and -65 for CR1, CR2, CR3, and CR4 areas, respectively. Considering the presence of sulfate in all four soil types and the value of the redox potential, a score of 5 is assigned for all four regions.14
With respect to the results in Table 2, due to the presence of sulfides in the soils of all areas, a rating of 3.5 is considered for this property. Although soil moisture content is low according to the results of experiments and the annual rainfall is low for the central desert regions of Iran (about 5 mm/year), due to poor drainage, a score of 1 is assigned to all areas.
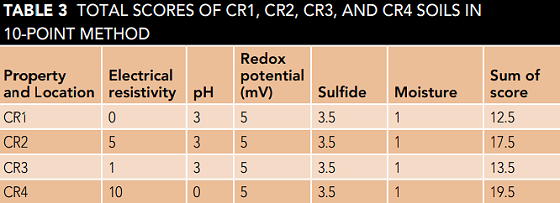
Table 3 shows the sum of soil scores for all 4 points, CR1, CR2, CR3, and CR4. As it is known, the sum of the soil scores of all four zones is higher than 10, thus being classified as a corrosive soil.
For accurate evaluation, the results of the 10-point method should be compared with the corrosion results of field and electrochemical experiments. After completing the burial period of specimens in the soil, the coupons were removed from the burial site steel and were cleaned with a hard bristle brush and washed with distilled water to remove the soil and surface oxides.
The weight loss results of the samples obtained from field corrosion experiments are shown in Table 4. Δm1, Δm2, and Δm3 lost weight after 45, 90, and 180 days, respectively. The corrosion rate of the specimens is calculated as mmpy according to NACE TM0169.6
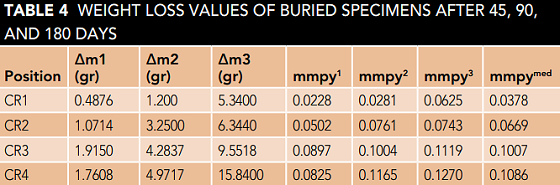
The values of mmpy1, mmpy2, and mmpy3 are corrosion rates of the specimens (mm/y) for periods of 45, 90, and 180 days, respectively. Given the time periods of 45, 90, and 180 days and the weight loss values obtained, mmpy values, and their average
mmpymed for these time periods are calculated according to Table 4.
Although weight loss tests can provide a detailed field assessment of the corrosion rate, they require long exposure times and the estimated corrosion rate is the average corrosion rate during the exposure time. Electrochemical measurements permit the determination of instant corrosion rates at a particular time.
Table 5 shows the potentiodynamic polarization results for the soils of the four regions. As shown in Table 5, the lowest Ecorr (-656 V) and the highest Icorr (8.49 µA/cm2) are measured in CR4 soil solution, which means that the corrosion resistance of St 37 steel in the CR4 soil environment is lower than the other three environments and thus the corrosion is more severe.
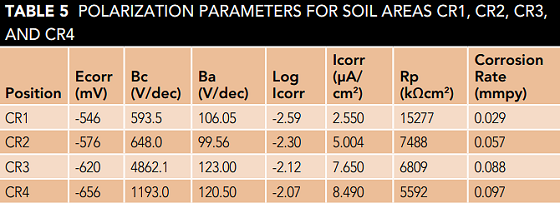
The polarization resistance (Rp) can be considered as a function of clay content. The increment in Rp due to a decrease in clay content is well observed for CR4, CR3, CR2, and CR1 soils, respectively, which means that the corrosion current decreases and as a result soil corrosivity reduces. Corrosion current (Icorr), which is directly related to corrosion rate, decreases for the soils of areas CR4, CR3, CR2, and CR1, respectively.15 The results show that the electrochemical experiments are in good agreement with weight loss test results.
Conclusion
In this study, soils corrosivity assessments of the central desert regions of Iran were investigated by the 10-point method. One of the most influential parameters in soil corrosivity is electrical resistance. This value is equal to 48.7 and 4.6 Ω.m for the soils of CR1 and CR4 zones with the lowest and highest corrosion rates, respectively, which indicated an inverse relationship between soil resistivity and corrosion rate. The trend of change in the intensity of the corrosion current is decreasing for CR4, CR3, CR2, and CR1, respectively.
† Trade name.
References and About the Authors