For many years, thermal-sprayed aluminum (TSA) has effectively protected offshore structures from the corrosive effects of seawater and the marine environment. Thermal spray, also known as metalizing, is a process that uses an energy source to melt metal in powdered or wire form, and then propel or spray small molten or softened metal particles onto the surface of another metal or structure.1 When they impact the substrate, the hot metal particles flatten and form pancake-shaped “splats.” Many thermal-spray processes are available, including electric arc spray, flame spray, plasma spray, high-velocity oxy-fuel spray, detonation gun deposition, and cold spray. The process selected depends on the desired coating properties, component size, and cost.2
According to NACE International member Shiladitya Paul with TWI (Cambridge, United Kingdom), TSA coatings are applied on subsea assets to protect carbon steel (CS) from corrosion because they act as a barrier as well as provide sacrificial protection. In seawater, aluminum has a low self-corrosion rate and is also anodic to CS. Because the galvanic potential of aluminum is more active than CS, TSA acts as an anode and provides a substantial level of cathodic protection (CP) at ambient and elevated temperatures. “TSA is essentially an evenly distributed anode,” Paul says. “Instead of having anodes attached to the structure at specific points, you have a distributed anode that is a 250- to 300-micron thick layer. When you minimize the steel surface that is exposed, you drastically minimize the cathode area; and the dissolution rate of your anode, in this case the TSA coating, is reduced quite a lot.”
Even when the TSA coating is damaged, corrosion of the CS substrate is not accelerated. The aluminum in the coating polarizes the CS in seawater to potentials in the protective range: –800 mV or below vs. a silver/silver chloride (Ag/AgCl) seawater reference electrode. The polarization process also promotes a calcareous deposit on exposed steel that reduces the cathode area and, as a result, decreases the corrosion rate; however, dissolution of the aluminum in the coating will occur during this activity.
Subsea gas pipelines account for 45% of natural gas exports to Europe, and in some areas the seabed can be several kilometers deep.3 In deep sea environments, a TSA coating can sustain damage from movement of the seabed that can cause wear, or from the impact of surrounding geological features, such as rocks. Paul notes the behavior of TSA-coated CS in natural seawater—with and without external CP—has been studied. The mechanism responsible for calcareous deposit formation on CS in seawater due to CP, and the influence of parameters such as temperature, pH at the steel/seawater interface, and the various ions present in seawater, have been examined as well, although the research has typically focused on calcareous deposit formation under ambient pressure and ambient or low temperatures with the polarization stemming from galvanic CP or impressed current CP rather than a TSA coating. Not much has been reported on the corrosion performance of TSA when it is damaged, and data on the performance of damaged TSA under the level of pressure experienced in deep seawater are virtually nonexistent.
“The solubility of the constituents that form these calcareous compounds is dependent on pressure,” Paul explains. “When using a thermally sprayed aluminum in very high pressure environments, we don’t know if the deposits that form will be protective or not if the coating is damaged.” To address these knowledge gaps, Paul conducted a research project to study the protective performance of TSA in deep seawater. In CORROSION 2017 paper no. 9009, “Protection of Deep Sea Steel Structures Using Thermally Sprayed Aluminum,” he reports the results.
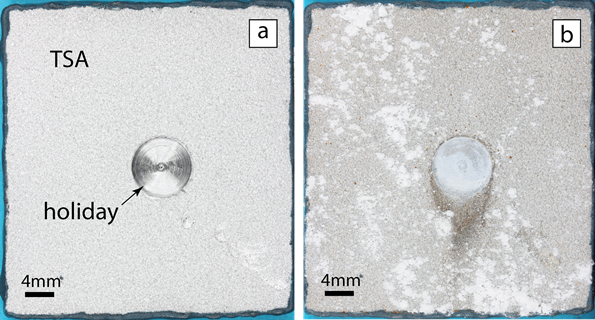
For the study, a flat 42- by 40- by 6-mm coupon made of carbon-manganese steel (BS EN 10025 Grade S355J2+N), which is often used in offshore environments, was thermally sprayed on one side with commercially pure aluminum (99.5% Al) using a twin-wire arc spray to deposit a TSA coating with a nominal thickness of 300 µm. A defect (holiday) amounting to 3% of the sample area was created by drilling through the entire thickness of the TSA coating and exposing the underlying CS substrate.
In an autoclave, the sample was exposed to synthetic seawater (per ASTM D11414) at 5 °C for 90 days at 50 MPa to simulate water pressure at a depth of 5,000 m. Since water pressure increases ~1 atm (the atmospheric pressure at sea level) for every 10 m of water depth, the pressure at a depth of 5,000 m is ~500 atm or 500 times greater than the pressure at sea level.5 After the autoclave exposure, the coated surface of the specimen was examined by light optical microscopy, scanning electron microscopy (SEM), and energy dispersive x-ray spectroscopy (EDX). The corrosion rate was calculated by determining the amount of dissolved aluminum in the seawater after testing. Since aluminum and iron salts were not originally present in the synthetic seawater, all Al3+ and Fe3+ ions in the solution after testing were presumed to originate from the sample.
Photographs of the TSA-coated specimen before and after exposure to the synthetic seawater indicate the exposed steel in the defect (holiday) region did not exhibit signs of any iron-based corrosion products from the steel; however, white corrosion product—aluminum hydroxide [Al(OH)3] from the TSA—was evident on the surface of the intact TSA coating. SEM of the holiday cross section showed a calcareous scale deposit on the exposed steel surface that varied from a few hundred µm near the TSA interface to few tens of µm near the center of the holiday region, and also confirmed there were no visible steel corrosion products. A detailed examination using EDX showed the protective multilayered deposit was comprised of magnesium and calcium compounds.
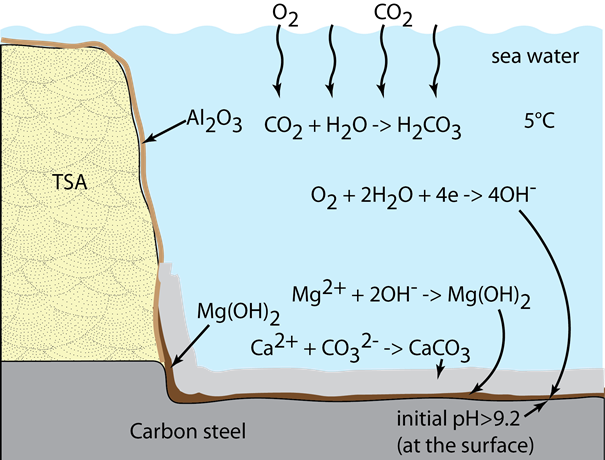
According to Paul, the aluminum in the TSA coating acts as the anode and polarizes the exposed steel surface in the holiday area. The polarization of exposed steel leads to the production of hydroxyl ions (OH-), which increase the pH at the steel/seawater interface. In turn, the OH- ions and high pH (>9) facilitate the precipitation of magnesium hydroxide [Mg(OH)2] from the seawater. “Seawater has a lot of magnesium and calcium ions, and the precipitation of one or the other depends on their relative solubility,” Paul explains. “If the solubility of the compounds in the seawater is sensitive to local pH, then they will precipitate out at certain pH levels and form the deposits.”
He notes that the composition of the calcareous deposits formed on a cathodically polarized steel surface also depends on temperature. The Mg(OH)2 is deposited at low temperatures and ambient pressures (≤5 °C) while the calcium carbonate (CaCO3) deposits are formed at ambient temperatures (20 to 25 °C) and ambient pressures. With increasing temperatures, he says, solubility of CaCO3 in water decreases while solubility of Mg(OH)2 increases.
During the 90-day test, as the Mg(OH)2 precipitated at the steel/seawater interface, the pH away from the interface decreased to a value where Mg(OH)2 could no longer precipitate. Therefore, the calcareous deposit next to steel consisted of a ~200-µm thick layer of predominantly of Mg(OH)2, while the deposit formed away from the steel was mainly CaCO3. This deposit behavior is very similar to calcareous deposits observed for ambient temperatures and pressures. The formation of calcareous deposits on exposed steel surfaces during CP of subsea structures is generally regarded as favorable since it reduces the cathode area and consequently reduces the corrosion of exposed steel, Paul says.
There was no evidence of CS corrosion products in the calcareous deposits, Paul adds, which led to the conclusion that it is very likely the damaged TSA will protect a CS surface from corrosion under the pressure conditions found in deep seawater.
Using data from the 90-day test, the calculated corrosion rates were 0.0002 mm y-1 for the aluminum in the coating and 0.002 mm y-1 for CS substrate. Paul comments that these values are low and the uncertainty and complexity associated with extrapolating corrosion rates from dissolved ion concentration data alone over a short test period dictates that the values should be treated with caution. Nonetheless, he says, formation of calcareous matter in the holiday region and the high integrity of the TSA coating indicates that it can potentially provide long-term corrosion protection to offshore CS structures in deep sea environments, even when the TSA coating is damaged in certain regions.
Contact Shiladitya Paul, TWI—email: Shiladitya.Paul@twi.co.uk.
References
1 “What is T.S.A?” Metalink Metallizing Systems, Apr. 30, 2010, http://www.metalinkwear.com/assets/knowledgeBase/What%20is%20TSA.pdf (July 6, 2017).
2 N. Ce, S. Paul, “Thermally Sprayed Aluminum Coatings for the Protection of Subsea Risers and Pipelines Carrying Hot Fluids,” Coatings 6, 58 (2016).
3 “How are subsea gas pipelines built?” Gasprom Informatorium, http://www.gazprominfo.com/articles/undersea-tubes/ (July 6, 2017).
4 ASTM D1141-98(2013), “Standard Practice for the Preparation of Substitute Ocean Water” (West Conshohocken, PA: ASTM International, 2013).
5 “Water Pressures at Ocean Depths,” National Oceanic and Atmospheric Administration, https://www.pmel.noaa.gov/eoi/nemo1998/education/pressure.html (July 6, 2017).