In countries with inadequate access to fossil fuels, energy from geothermal power plants provides a crucial service. In fact, right now the Asia-Pacific region supplies over one-third of the world’s geothermal energy production. Geothermal power plants produce electricity using the natural heat of the earth, which is accomplished by:
• Injecting cold water into hot rock (usually >220 °C).
• Pumping hot water from the earth and using the heat to create steam.
• Using steam to run turbines and generators.
Figure 1 illustrates this process.
The high temperatures of geothermal wells cause difficulties relating to scale control regarding both inhibition performance and thermal stability. These limitations reduce the chemistry of scale inhibitors that can be used in these extreme environments. Geothermal brines often contain dissolved minerals and gases that can cause deposition in wells and on topside equipment, the presence of which can lead to numerous problems.
The conditions and brine chemistry can differ significantly from plant to plant, but generally, the high-temperature conditions are conducive to calcium carbonate—the most common type found in geothermal systems—along with silica/silicate, and iron sulfide scale deposition. The calcium carbonate control phosphonates usually offer better inhibition capability and increased brine tolerance over polymers and is more cost efficient. The thermal stability limits, typically 170 to 200 °C, for low molecular weight phosphonates is generally not high enough for geothermal wells. Therefore, a need exists for producing a novel phosphonate chemistry that can perform up to 250 °C.
Researchers Bethanni McCabe and Stephen Heath1 studied a geothermal plant with a low salinity brine and temperature of 250 °C located in the Pacific region. They focused on developing a new, biodegradable phosphonate with a high calcium tolerance that can perform in difficult conditions.

To test thermal stability, the researchers considered a sample stable if no signs of precipitation or separation took place and deemed darker coloration acceptable, provided that one single phase is observed. They aged the phosphonate in an undiluted solution at 250 °C for 24 h. It was then removed, cooled under pressure, and was visually inspected against an untested product (Figure 2). No sign of degradation or separation occurred, and while the sample appeared slightly darker after the aging process, no solids or phase separation was visible.
The researchers tested the efficiency of phosphonate against calcium carbonate scale utilizing a dynamic scale loop (DSL) test (Figure 3). The conditions of the test included a temperature of 250 °C, 250 psi of pressure, a flow rate of 10 mL/ min, a 1-m coil length, and a 0.0625-in (1.5875-mm) internal diameter coil. This technique is common; however, testing at 250 °C meant that the researchers were at the high end of the temperature testing parameters. This necessitated some alterations to the rig to maintain safety.
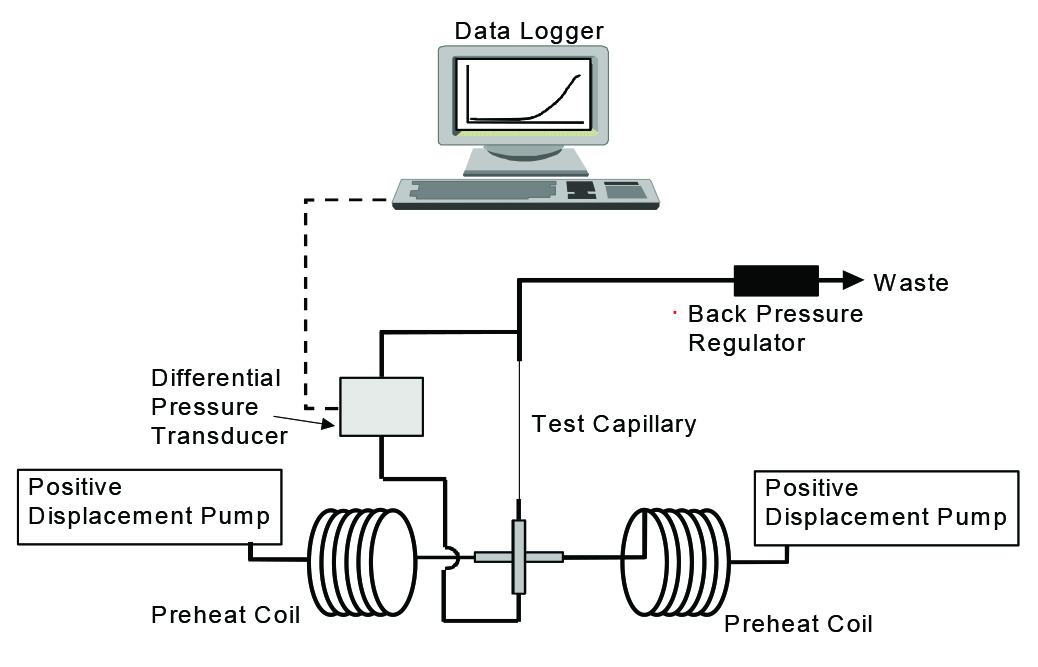
Two synthetic brines, one containing anions and one containing cations, were prepared. The brines were pumped into a mixing chamber, and flowed through the coil, where differential pressure was determined. To meet the minimum inhibitor concentration standards, no increase in differential pressure should occur during the test period.
As a comparison, the researchers also tested a sulfonated copolymer and a low molecular weight polyphosphino carboxylic acid (PPCA). The results revealed that the phosphonate had a minimum inhibitor concentration of 5 ppm, the sulfonated copolymer had 50 to 100 ppm, and the low molecular weight PPCA had 10 ppm. At these dosages, no calcium carbonate formation occurred due to the lack of increase in differential pressure. These findings reveal that the novel phosphonate protects against calcium carbonate at these high temperatures better than the polymeric scale inhibitors.
Additional testing was performed to compare the original phosphonate to the thermally aged sample. There was no differential pressure increase throughout the 90-minute test, showing that the aging process did not affect the inhibition performance and that no scale formed. The novel phosphonate was, again, able to perform well under extreme environments.
Calcium tolerance is considered as a chemical’s capability to maintain solubility in the presence of high levels of calcium. In general, phosphonates at elevated temperatures possess a low tolerance for calcium. If phosphonates bind with calcium, the phosphonates are used up, and none are left to perform as scale inhibitors. The researchers note that it is important that this novel phosphonate be tolerant to high levels of calcium and high temperatures, as seen in the geothermal system. It was tested for compatibility in a synthetic brine, which was prepared with the removal of scaling ions, sulfate, and bicarbonate. The scale-forming ions were eliminated to exclude the potential for scale formation to be mistaken for incompatibility.
The product was assessed for compatibility with a high calcium brine at 120 °C and tested for a 24-h period up to a dosage of 1%. Occurrence of liquid phase separation or precipitate would be regarded as a fail; however, it was found to be fully compatible at all doses, showing no precipitation. The novel phosphate can be considered to have a high calcium tolerance, and able to remain soluble at 30,000 ppm of calcium present in the brine (Figure 4). No comparative testing was performed with other scale inhibitors, but most scale inhibitors are known to be intolerable to such high levels of calcium and elevated temperatures.
It is imperative that chemicals used in geothermal applications be environmentally friendly. Governmental pressure is increasingly pushing toward greener chemistries, which creates a challenge to find products that can withstand the extreme environments found in geothermal systems and also be biodegradable.
The biodegradability of the novel phosphonate was evaluated to gauge its suitability for use in Norway. A closed-bottle method was used, involving a predetermined amount of chemical being dissolved into seawater. The solution remained in dark conditions and a constant temperature was maintained. Dissolved oxygen analysis was carried out at specific desired time periods. The results showed that the product is considered biodegradable because testing showed greater than 20% biodegradation after 28 days of testing.
The researchers conclude that the novel phosphonate chemistry is useful in ultrahigh geothermal applications. Its high tolerance to calcium, biodegradability, thermal stability, and good performance against calcium carbonate scaling prove valuable in a variety of environments.
Reference
1 B. McCabe, S. Heath, “Development of a Novel Phosphonate Scale Inhibitor for Scale Control in Geothermal Applications,” CORROSION 2019, paper no. 13275 (Houston, TX: NACE International, 2019).