A new study from university researchers finds significant increases in both salinization and alkalinization at U.S. stream and river sites, which can make water more corrosive to pipes and lead to problems such as those that occurred earlier this decade in Flint, Michigan.
When Flint switched its primary water source to the Flint River in 2014, the river’s high salt load, combined with chemical treatments, made the water more corrosive, which caused lead to leach from water pipes and create that city’s well-documented water crisis.1
In the aftermath of that crisis, researchers at the University of Maryland (UMD) (College Park, Maryland, USA) and several other institutions studied survey monitoring sites across the country over the past 50 years. In analyzing the data, the researchers found major increases in both salinization and alkalinization, with results suggesting that the combination of different salt compounds such as road deicers and fertilizers can do more damage than any one salt on its own.
“We created the name ‘Freshwater Salinization Syndrome’ because we realized it’s a suite of effects on water quality, with many different salt ions linked together,” says Sujay Kaushal, a UMD professor of geology and lead author of the study.2 “We didn’t know that before. Many people assume that when you apply salt to the landscape it just gets washed away and disappears. But salt accumulates in soils and groundwater and takes decades to get flushed out.”
As part of the research, Kaushal says he finds a need to better monitor and replace aging water pipes throughout the country that have been impacted by corrosion and scaling, such as those in Flint—or through the buildup of mineral deposits and microbial films. Such pipes are particularly vulnerable to saltier, more alkaline water, which can exacerbate the release of toxic metals and other contaminants.
“The trends we are seeing in the data all suggest that we need to consider the issue of salt pollution and begin to take it seriously,” Kaushal says. “The Environmental Protection Agency [Washington, DC, USA] does not regulate salts as primary contaminants in drinking water at the federal level, and there is inconsistency in managing salt pollution at the local level.”
Kaushal’s team at UMD was supplemented by researchers at the Cary Institute of Ecosystem Studies (Millbrook, New York, USA), the University of Connecticut (Storrs, Connecticut, USA), the University of Virginia (Charlottesville, Virginia, USA) and Chatham University (Pittsburgh, Pennsylvania, USA).
Monitoring Sites
In their study, the researchers assessed long-term changes in freshwater salinity and pH at the continental scale by drawing on data recorded over the past 50 years at 232 U.S. Geological Survey (Reston, Virginia, USA) monitoring sites located across the United States.
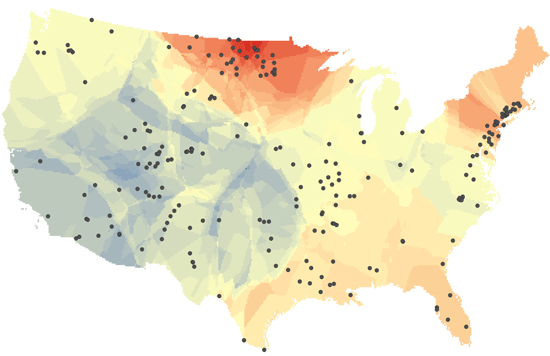
The researchers say they documented sharp chemical changes in many of the country’s major rivers, including the Mississippi, Hudson, Potomac, Neuse, Canadian, and Chattahoochee. Many of these rivers supply drinking water for nearby cities and towns, including some of the most densely populated urban centers along the U.S. East Coast.
According to Kaushal, most previous freshwater salinization research has focused on sodium chloride (NaCl), which is the dominant chemical in road deicers. But in terms of chemistry, he notes that salt has a broader definition, encompassing any combination of positively and negatively charged ions that dissociate in water. Some of the most common positive ions found in salts—including sodium (Na), calcium (Ca), magnesium (Mg), and potassium (K)—can have damaging effects on freshwater at higher concentrations. “These ‘cocktails’ of salts can be more toxic than just one salt, as some ions can displace and release other ions from soils and rocks, compounding the problem,” Kaushal said. “Ecotoxicologists are just now beginning to understand this.”
Varying Causes by Region
The current study is the first to simultaneously account for multiple salt ions in freshwater across the United States and southern Canada. The results suggest that salt ions are driving up the pH of freshwater as well, making it more alkaline. Over the time period covered by the study, the researchers concluded that 37% of the drainage area of the contiguous United States experienced a significant increase in salinity. Alkalinization, which is influenced by many factors in addition to salinity, increased by 90%, they say.
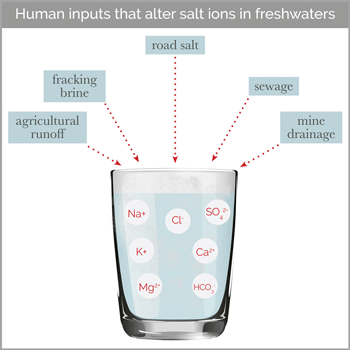 “Our study is the first to document a link between increased salinization and alkalinization at the continental scale,” says study co-author Gene Likens, president emeritus at Cary and a distinguished research professor at Connecticut. “Until now, we didn’t fully appreciate the role that different salts play in altering the pH of streams and rivers of our country. Salt content and pH are fundamental aspects of water chemistry, so these are major changes to the properties of freshwater.”
“Our study is the first to document a link between increased salinization and alkalinization at the continental scale,” says study co-author Gene Likens, president emeritus at Cary and a distinguished research professor at Connecticut. “Until now, we didn’t fully appreciate the role that different salts play in altering the pH of streams and rivers of our country. Salt content and pH are fundamental aspects of water chemistry, so these are major changes to the properties of freshwater.”
The root causes of increased salt in waterways vary from region to region, Kaushal says. In the snowy Mid-Atlantic and New England, they explain that road salt applied to maintain roadways in winter is a primary culprit. Meanwhile, in the heavily agricultural Midwest, fertilizers—particularly those with high potassium content—make major contributions. In other regions, mining waste and the weathering of concrete, rocks, and soils can release salts into adjacent waterways, the researchers explain.
“We found that the pH of some rivers started increasing in the 1950s and 60s—decades before the implementation of acid rain regulations,” says Michael Pace, a professor of environmental sciences at Virginia and a study co-author. “We also observed increased salt concentrations in the Southeast, where they don’t apply road salts. These surprising trends presented a puzzle that our team worked together to solve.”
Strategies for Improvement
Historically, salt concentrations have been very high in the desert region of the U.S. Southwest. But in their study, the researchers actually documented an overall decrease in salinity over time. The researchers attribute this decrease to a variety of factors, including changes in land and water use, coupled with an effort on the part of state and local governments to reduce salt inputs and improve water resource management strategies.
Kaushal notes that many strategies for managing salt pollution already exist. For example, evidence suggests that brines can be more efficient than granulated salt for deicing roads, yielding the same effect with less overall salt input. Salting before a major snow event can also improve results. Kaushal says many cities and states in the Mid-Atlantic and Northeast regions have outdated and inefficient salt-spreading equipment that is overdue for an upgrade.
“Also, not all salts are created equally in terms of their ability to melt ice at certain temperatures,” Kaushal adds. “Choosing the right salt compounds for the right conditions can help melt snow and ice more efficiently with less salt input, which would go a long way toward solving the problem.”
The researchers note similar issues with applying fertilizers in agricultural settings. In many cases, applying the right amount of fertilizer at the right time of the season can reduce the overall output of salts into nearby streams and rivers, they say. The researchers also note that more careful urban development strategies—in particular, building further from waterways and designing more effective stormwater drainage systems—can help reduce the amount of salt washed away from weathered concrete.
“As a society, we’re addressing the water quality issues of sewage, wastewater, and nutrient loading,” says Tom Torgersen, director of the water sustainability and climate program at the U.S. National Science Foundation (NSF) (Alexandria, Virginia, USA), which funded the research. “But our impact on water quality remains significant as a result of our increasing population, the size of our built infrastructure, and other factors. Management of water quality impacts remains a challenge.”
Source: University of Maryland, www.umd.edu. Contact Sujay Kaushal, University of Maryland—email: skaushal@umd.edu.
References
1 K. Riggs Larsen, “The Science Behind It: Corrosion Caused Lead-Tainted Water in Flint, Michigan.” MP, June 7, 2016, http://www.materialsperformance.com/articles/material-selection-design/2016/06/the-science-behind-it-corrosion-caused-lead-tainted-water-in-flint-michigan (Feb. 12, 2018).
2 “North American Waterways are Becoming Saltier and More Alkaline,” University of Maryland College of Computer, Mathematical, and Natural Sciences, News & Events, Jan. 8, 2018, https://cmns.umd.edu/news-events/features/4059 (Feb. 12, 2018).