The Matanzas River in St. Johns County is a narrow, 23-mile (37-km) long saltwater estuary located just off the northeastern coast of Florida in the United States. It is sheltered from the Atlantic Ocean by a barrier island, Anastasia Island. To connect Anastasia Island with the mainland, two major bridges cross the Matanzas River—the Bridge of Lions in St. Augustine and the Mickler-O'Connell Bridge located just south of St. Augustine. Currently the Mickler-O’Connell Bridge, which is experiencing corrosion of its steel H-piles, is being studied by the Florida International University (FIU) (Miami, Florida, USA), at the request of the Florida Department of Transportation (FDOT) (Gainesville, Florida, USA), for the possibility of microbiologically influenced corrosion (MIC).
The Mickler-O'Connell Bridge, built in 1976, carries an average of 18,750 vehicles daily. It is a typical concrete beam structure on footings/columns/strut piers with 5.5 ft (1.7 m) steel girders on the three main channel spans. Each of its 14 footings is supported by seven to nine concrete piles along with two uncoated steel H-piles. According to NACE International member Matthew Duncan, corrosion mitigation technologist with FDOT, divers conducting bridge inspections in 2000 detected heavy corrosion of the steel H-piles that other bridges in that area were not experiencing. To mitigate the corrosion on the H-piles, the FDOT’s corrosion group decided at that time to implement a galvanic cathodic protection (CP) system using bulk zinc anodes.
The CP system, installed and energized in 2006, included an anode string for each of the bridge’s nine piers. Seven anode strings were comprised of three 50-lb (23-kg) zinc anodes cast onto a galvanized cable, and two anode strings consisted of two 50-lb zinc anodes on a galvanized cable (for H-piles with a small amount of seawater exposure between the mudline and the footing). The bottom ends of the anode strings were anchored into the mudline.
High Anode Consumption and Continued Corrosion
The CP system was monitored, and after one year of service, the FDOT corrosion group observed a high consumption rate of the anodes. During a biannual visual inspection, it was verified that the anodes were depleting faster than anticipated. In 2008, the FDOT corrosion group determined that the galvanic CP system was inadequate. Corrosion on the H-piles had continued. The corrosion was not generalized—it did not cover the entire surface of the steel. Instead, it appeared as localized sections of pitting and holes on the H-piles’ web and flanges. The corrosion pitting and holes were discovered in water depths that ranged from ~1 to 30 ft (0.4 to 9 m) below the pile cap, with a median water depth of 2.5 ft (0.8 m). A large percentage of the deficiencies occurred close to the water surface. Most of the corrosion pits were 0.125 in (3 mm) in diameter, but pits with diameters up to 0.5 in (12 mm) and corrosion holes with diameters as large as 3 in (76 mm) were recorded. The corrosion cells/pits were covered with a bright orange plume. When the plume was removed, flakey, grey/black corrosion product was found underneath. In 2009, the galvanic CP system was replaced with an impressed current CP system designed to protect the H-piles as well as remediate footings, columns, and struts on selected piers.

“We were surprised because we thought the CP system would be sufficient,” Duncan says, noting that the magnitude of the galvanic anode consumption was quite significant.
Marine environments are notorious for supporting aggressive corrosion. For bridges, it’s the presence of chloride ions that typically creates corrosion problems for steel structural elements as well as steel reinforcement embedded in concrete. Chloride contamination can affect bridge elements submerged in seawater, as well as those exposed to the tidal zone, the splash zone above high tide, and the atmospheric zone.1 However, discussions on the corrosion found on the H-piles of the Mickler-O’Connell Bridge led the FDOT corrosion group to consider the possibility that the damage was being caused by MIC. Traditionally, MIC has not been a major degradation concern for Florida coastal and inland bridges; however, the severe corrosion on the steel bridge piles presented strong evidence of microbial activity.
Microorganisms can adhere to most surfaces in contact with natural waters, according to researchers at FIU. For the microorganisms to survive, the availability of nutrients is essential. The water needs to contain suitable forms of carbon, hydrogen, oxygen, sulfur, phosphorus, potassium, magnesium, calcium, manganese, nitrogen, and other elements to support microbial growth and biofilm formation. Many microorganisms, when they reproduce, create exopolymers that influence the chemistry on the surface where they are attached. MIC, an electrochemical process where corrosion is influenced by microorganisms—usually through the interaction of a biofilm with the metal surface—is known to degrade materials in a variety of industries.
According to NACE International member Samanbar Permeh, a scientist with FIU, a biofilm creates an internal environment with physical and chemical properties, such as pH, dissolved oxygen (DO), etc., that may be significantly different than the environmental properties outside of the biofilm. This causes changes in the electrochemistry of the biofilm/metal interface and can accelerate corrosion. Research has shown that 20% of corrosion costs on marine steel infrastructure such as bridges, wharfs, platforms, and pipelines is due to microorganism activity. A large portion of these corrosion costs are attributed to sulfate-reducing bacteria (SRB) that form biofilms on iron and steel, Permeh adds.
When the Mickler-O’Connell Bridge was inspected in 2014, the inspectors noted that all 28 H-piles had suffered further corrosion and were rated as being in poor condition. Random, dense patterns of corrosion cells and pits were present and a bright orange plume again covered flakey, grey/black corrosion product. The bare metal underneath was bright, and several holes (with diameters up to 3 in) were found on the web and flanges. Some of the corrosion cells and pits were located at the flange/web interface.
Microbiologically Influenced Corrosion Is Suspected
To determine if MIC is responsible for the degradation of the steel H-piles, Permeh and NACE member Kingsley Lau, as well as other scientists at FIU, are working with Duncan and the FDOT corrosion group to research the corrosion found on the Mickler-O’Connell Bridge H-piles. The investigation will determine the bacteria present, nutrient levels, environmental conditions, and other factors at the bridge site that could support MIC.
“You have to do a test to find out which nutrients and other components are in the water that support MIC,” explains Lau. “It’s a multistep process. We’re not 100% positive, but there is a strong likelihood the MIC is causing the corrosion problem.”
During a visit to the bridge site in 2016, water samples were collected from two locations at two depths (10 and 20 ft [3 and 6 m]) below high tide (BHT). Underwater photographs were also taken. Water analysis was performed to determine if microorganisms were present as well as characterize the water’s environmental conditions (i.e., DO, pH, conductivity, sulfate, chloride, phosphorus, nitrate, total organic nitrogen, total nitrogen, ammonia, and iron content) to establish whether it could support the microorganisms related to MIC.
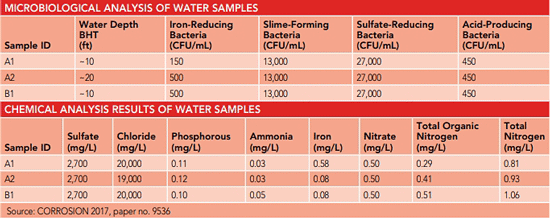
Earlier assessments in 2013 using steel coupons and water samples from the bridge site had determined the presence of bacteria often associated with MIC. Bacteria found on steel coupons included a large number of anaerobic SRB (>100,000 colony forming units [CFU]/mL), and smaller numbers of acid-producing bacteria (APB) and slime-forming bacteria (SFB). Water sample analysis also indicated the presence of anaerobic SRB (from ~100 to 10,000 CFU/mL), APB, and SFB.
Lau comments that testing on site showed that all the bacterial culprits responsible for MIC were still present. Similar to the earlier test results in 2013, SRB, APB, and SFB were identified in all the water samples tested in 2016. There was a high accumulation of SRB (~27,000 CFU/mL) at both depths at both test locations. The researchers note it is understood that the quantity of bacteria identified in the water samples cannot directly correlate to MIC risk; however, the high concentrations indicate there is a greater possibility for the bacteria to proliferate and contribute to MIC. Analyses of the water samples also confirmed the presence of important nutrients such as sulfate, phosphorous, nitrogen, and iron that can support a large bacteria colony.
“They [bacteria] can be prolific in this area, and the environment sustains their growth. We can put two and two together and say that MIC can happen on this site. Whether those holes are caused by MIC? It can be likely,” Lau says, noting that MIC may be compounded by heavy marine growth, which could intensify its effect.
Lau acknowledges that more research is needed to determine if MIC is causing the metal loss and degradation of the H-piles and how to mitigate the corrosion and prevent holes from occurring. One big question the researchers want to answer is the effect of marine growth, since it’s so abundant at this site. If the marine growth is heavy, will it enhance the proliferation of bacteria that support MIC?

Part of the research project will focus on macrofouling—the adhesion of barnacles and other marine life—on the steel, and how it is associated with the bacteria present and MIC. Macrofouling organisms use bacteria to seed themselves on surfaces, and the H-piles had a large amount of marine growth. Additionally, macrofouling adhered to the H-piles created a crevice environment, which is corrosive in nature. “What we’re trying to address is the effect from crevice environments that form with macrofoulers, and can they support MIC,” Lau adds.
Crevice corrosion is a type of attack associated with small volumes of stagnant water or moisture found near holes, gaskets, lap joints, bolts, rivets, under insoluble deposits and disbonded coatings, and in other crevice-like areas such as under macro- and microbiological colonies. The crevice must be wide enough for reactants to enter the corrosion site, yet sufficiently narrow to contain stagnation in the crevice area. Initially the corrosion mechanism follows the same process as general corrosion, where the metal loses electrons as it corrodes at the anode. Those electrons lost by the metal are consumed at the cathode, typically by the reduction of oxygen. With time, crevice corrosion can reduce the pH in the crevice, which further accelerates the corrosion process.1
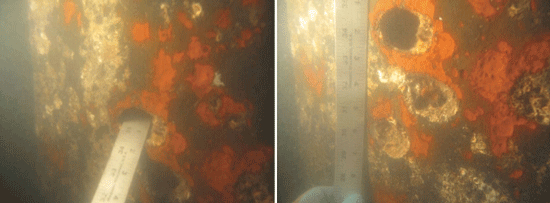
“We can’t rule out the effect of marine growth because it is so significant and can create a local environment,” Lau says, noting that macrofouling along with the presence of MIC is thought to cause localized heavy corrosion. “First, we want to understand if MIC is present underneath the macrofouling in the crevice environment. If we can determine that, we will have a better direction to turn in terms of mitigation,” he adds.
Mitigating the Corrosion
The CP system used on the H-piles interests the scientists. They theorize that one reason why the corrosion damage is so considerable on the H-piles may be an inadequate level of CP. Although the level of CP on the pile was in the accepted polarization range, higher levels of CP—polarization potentials that are more negative than –950 mV vs. a copper/copper sulfate (Cu/CuSO4) reference electrode (CSE)—are typically required in the presence of MIC. “We’re trying to address that at the site, especially since we have a presence of macrofouling that may shield the protected surface from being sufficiently polarized,” says Lau.
The effectiveness of CP in this case also may be affected by the chemical composition of the macrofouling organisms, particularly barnacles. These creatures create a layer of calcium carbonate (CaCO₃) that forms crevices on the surface of the structure. When applying CP in any crevice environment, Lau explains, it is hard to cathodically polarize the environment within the crevice in a pit. In this case, the crevice is being formed by the marine growth, he says, and the CP is working to polarize the entire surface area underneath it. “It’s our feeling that underneath the macrofouling, in these crevice areas, MIC is prolific and that is why mitigation by CP may be reduced and less effective,” Lau adds. “We want to try more negative polarization and hopefully it will compensate for the problem with current distribution. This is a known problem with crevice environments.”

To identify the level of polarization that can mitigate MIC in the crevice environment caused by macrofouling, the researchers have created artificial crevices in the laboratory to represent what might be formed by sea life, and are polarizing those environments with CP. Additionally, they plan to bring in samples with existing marine growth from the bridge site. “We’re looking at the physical effects of macrofouling and how it acts as a shield and may affect the CP system,” Lau explains, adding that their research will also focus on SRB since the site had high sulfate concentrations that can support bacteria growth. Once the researchers determine the role of macrofouling in the corrosion of the H-piles, they can make recommendations on whether routine cleaning would reduce its effect on the steel.
In addition to CP, the researchers are also looking at coatings to mitigate the corrosion on the H-piles. One type, a commercially available antifouling coating with a copper-free biocide, would prevent the formation of a biofilm or settlement of macrofouling so cathodic polarization would be improved, says Permeh. This is particularly beneficial when you have a shielding effect from barnacles and other sea life, she adds. A polyurea coating also is being evaluated to determine its effect on biofilm formation. Polyurea, a 100% solids elastomer technology, is a highly elastic, waterproof coating that has been used in wastewater plants, which also have MIC problems associated with SRB.
There are many marine regions in Florida with environmental characteristics similar to the Mickler-O’Connell Bridge site and conditions that may sustain microbial activity and support MIC. The researchers took their work a step further to identify these areas. They compared results on water chemistry and microorganism content from the case study site tests with available environmental databases from Florida’s water management districts to identify locations with comparable conditions that are conducive to MIC. As a result, many sites with characteristics similar to the case study site were found. Verifying microbial activity at other sites and identifying the possibility MIC there is of interest, and further laboratory and field testing is ongoing.
Reference
1 “Corrosion Control Plan for Bridges,” NACE International white paper, November 2012, https://www.nace.org/uploadedFiles/Corrosion_Central/Corrosion_101/White_Papers/CorrosionControlPlanForBridges.pdf (November 8, 2017).
Bibliography
Permeh, S., C. Reid, M. Echeverria-Boan, K. Lau, B. Tansel, M. Duncan, and I. Lasa, “Microbiological Influenced Corrosion (MIC) in Florida Marine Environment: A Case Study.” CORROSION 2017, paper no. 9536. Houston, TX: NACE International, 2017.