Historically, corrosion in both conventional and unconventional oil and gas wells is attributable to either hydrogen sulfide (H2S), carbon dioxide (CO2), or a combination of the two. Because of this, much effort has been expended creating software models to predict such attack on steel equipment. Moreover, the typical corrosion products in these well environments are iron(II) carbonate (FeCO3), iron(II,III) oxide (Fe3O4), and various forms of iron(II) sulfide (FeS). However, recently some unusual results have appeared during the investigation of corrosion for shale wells in both the Permian Basin in Texas and New Mexico and the Eagle Ford in Texas. Several cases are cited herein to illustrate these unusual findings.
Case 1
Figure 1 shows the internal pitting attack on L80 tubing from a Permian Basin well that produces oil, water, and gas with 0.16% CO2 in the gas phase and no H2S. The bicarbonate content of the water was 49 ppm and the well had serious barium sulfate (BaSO4) scaling issues. The well produced up tubing for approximately four years, using gas lift, and then was converted to artificial lift for only a few months with an electric submersible pump (ESP).
The well was initially completed in April 2014, followed by a workover in January 2017, and then by another workover on July 20, 2018. Although the tubing was inspected after each workover, it was uncertain how adequate these inspections were; therefore, the possible corrosion rates based on each time interval were:
• Most recent workover: July 2018 = 6.0 mm/y (237 mpy)
• Previous workover: January 2017 = 2.2 mm/y (87 mpy)
• Initial completion: April 2014 = 1.0 mm/y (41 mpy)
 Flowing tubing pressures were low, and with such low CO2 content the corrosion rate from CO2 was not expected to be high. Using one of the standard corrosion software models, the corrosion rate was predicted to be 0.33 mm/y (13 mpy) at the top of the tubing where the corrosion occurred, and from which the section shown in Figure 1 was taken. Moreover, even adding up to 10,000 ppm H2S into the model, the H2S pitting rate was predicted to be only 0.38 mm/y (15 mpy).
Flowing tubing pressures were low, and with such low CO2 content the corrosion rate from CO2 was not expected to be high. Using one of the standard corrosion software models, the corrosion rate was predicted to be 0.33 mm/y (13 mpy) at the top of the tubing where the corrosion occurred, and from which the section shown in Figure 1 was taken. Moreover, even adding up to 10,000 ppm H2S into the model, the H2S pitting rate was predicted to be only 0.38 mm/y (15 mpy).
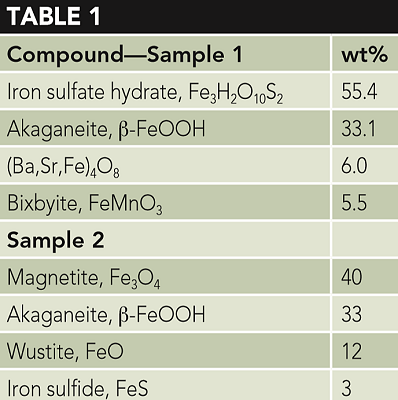
Corrosion products were removed from pits inside of the tubing and analyzed using x-ray diffraction (XRD) and energy dispersive spectroscopy. Table 1 presents the analysis of two samples.
The two samples were analyzed by two different laboratories. The presence of an unusual compound, iron sulfate hydrate (Fe3H2O10S2), prompted the second analysis. This iron sulfate hydrate is referenced in the XRD database.1 While the second analysis did not detect this compound, both analyses identified high concentrations of akaganeite (β-FeOOH), which will be discussed later. It should also be noted that no iron carbonate was detected.
Case 2
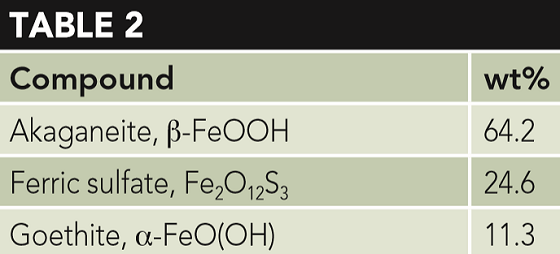
A well in the Eagle Ford shale, where the conditions were not provided, developed pitting in a short time on low alloy steel (AISI 4130) wellhead equipment. The XRD analysis from the pits are presented in Table 2.
It is interesting to note that neither FeCO3 nor FeS were present, which are common corrosion products in the Eagle Ford due to the presence, to some extent, of CO2 and H2S in every well. Furthermore, both ferric sulfate (Fe2O12S3) and akaganeite again appear in large quantities.
Case 3
After only 105 days run time of an ESP in another Permian Basin shale well, the tubing was pulled and found to have pitting on the order of 12.4 mm/y (490 mpy), again near the top of the tubing string. The CO2 content in the gas phase was 0.23% and corrosion modeling predicted a corrosion rate at this location of 0.38 mm/y (15 mpy). Table 3 shows the XRD results from a sample removed from the pits.
In this case FeCO3 does appear, but again, a large quantity of β-FeOOH exists, just as in the other cases. To reemphasize, corrosion modeling of this well did not support severe pitting from CO2 corrosion as a cause.

Discussion
The results of these investigations are inconsistent with CO2 corrosion, the expected source for pitting based on the gas analyses. In oil and gas producing wells, both for conventional and unconventional production, corrosion typically occurs from only two possible sources—CO2 and H2S. Corrosion products from the reaction of steel tubing and components with these two gases are either iron carbonate or iron sulfide, none of which were identified in these failures. In some cases, iron oxides, such as magnetite, form in CO2 environments, but only at much higher temperatures than were present in any of these cases.
Also, it is known that β-FeOOH forms in the presence of high chloride content environments. The deposits found in these failures are unique, especially the presence of large amounts of iron sulfate. This compound is atypical in any oil and gas wells, and thus, requires consideration of altogether unique sources of such compounds. While the role of sulfate-reducing bacteria (SRB) cannot be ruled out in these cases, the reactions from SRB in reducing sulfate to sulfide and the end product of iron sulfide is typically very rapid and commonly observed. A mechanism that would allow iron sulfate to form and stabilize from SRB is unknown at present.
A possible mechanism is proposed herein due to the oxidation of pyrite in the reservoir. Pyrite has been reported to be present in some formations in both the Permian and Eagle Ford reservoirs and oxidation by either oxygen that was injected at the time of fracking with the frack water and/or ferric ions that were oxidized from ferrous ions (corrosion).
Teevens2 has reported measurable dissolved oxygen (DO) on flowback/produced waters between 40 ppb to 1 ppm for as long as six years after fracking. This idea of DO in frack water returns is somewhat controversial but has never been properly studied so it cannot be discounted at this time. Furthermore, it is well known that frack waters contain large amounts of DO and often no attempt is made to remove this before fracking.
Oxidation of pyrite by oxygen (O2) and ferric ions has been extensively studied, especially for flowback water in the Marcellus shale. The chemical reactions are as follows:3
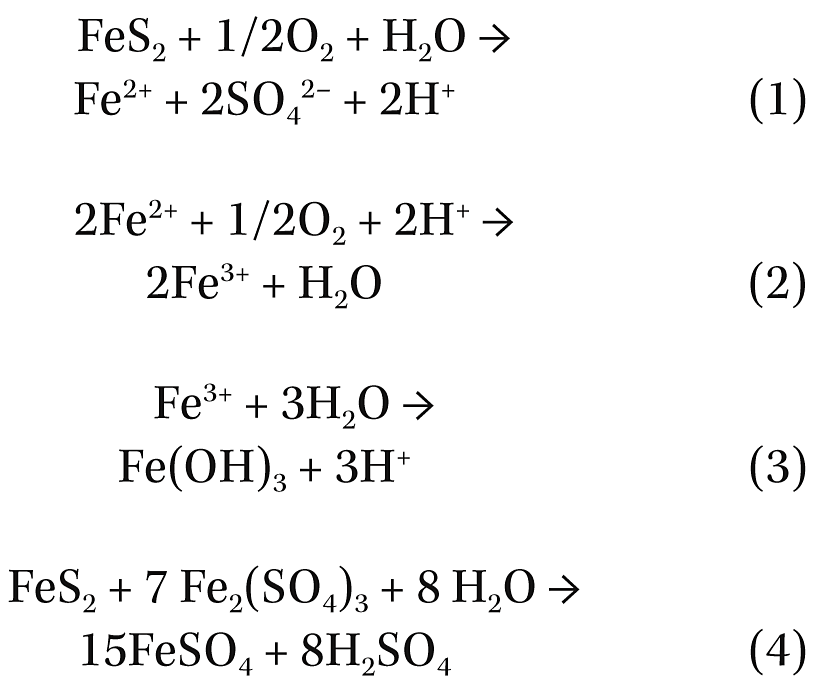
As determined by others,4 these reactions can occur at ambient temperature and near neutral pH; however, the primary oxidant at neutral and alkaline pH is O2. Fe(OH)3 is also represented as β-FeOOH (akaganeite) and has identical XRD peaks as schwertmannite [Fe8O8(OH)6SO4].5 The two compounds are isostructural.5 Subtle changes in crystallinity can move the peaks of these compounds, making differentiation difficult.
Thus, it can be seen that from the previous oxidation of pyrite, the observed iron sulfate and iron oxides (which also may be ferric sulfate) reported could be generated as well as sulfuric acid (H2SO4) that could increase the corrosion rate of steel. This suggests that CO2 corrosion may not be the operating mechanism for corrosion in low CO2 content wells. Furthermore, the presence of high contents of sulfate generated by these reactions could explain the unusually high barium sulfate problems encountered in some of these wells due to the presence of an abundance of sulfate.
In conclusion, it is proposed that the high corrosion rates in the cases shown here were from O2 and sulfuric acid in the flowback water, the latter produced by oxidation of pyrite. Although this possible mechanism is speculative, it is the only one that comports with the current evidence. If in fact this process does occur in shale wells, it could be quite unpredictable from a corrosion standpoint, and therefore, from a corrosion-control standpoint.
References
1 C.V. Subban, et. al., “Preparation, Structure and Electrochemistry of Layered Polyanionic Hydrosulfates: LiMSO4OH (M=Fe, Co, Mn) Electrodes for Li-Ion Batteries,” J. Chem. Soc. 135 (2013): pp. 3,653-3,661.
2 P. Teevens, private communication.
3 R.D. Vidic, “Sustainable Management of Flowback Water during Hydraulic Fracturing of Marcellus Shale for Natural Gas Production,” U.S. DOE Report, DE-FE0000975, April 2015.
4 C. Moses, J. Herman, “Pyrite Oxidation in Circumneutral pH,” Geochim. Cosmochim. Acta 55 (1991): p. 471.
5 S. Regenspurg, “Characterisation of Schwertmannite-Geochemical Interactions with Arsenate and Chromate and Significance in Sediments of Lignite Opencast Lakes” (Ph.D. diss., University of Bayreuth Dissertation, 2002).