A high percentage of maintenance costs in a petroleum refinery are due to failures caused by corrosion. The indirect costs of corrosion—unit shut downs, loss of productivity, and environmental pollution—can be even one order higher than the direct corrosion cost. Additionally, corrosion failures can be associated with accidents where the cost cannot be estimated.
According to Jigneshkumar J. Desai and Ashik S. Murthy with Fluor Daniel India Private, Ltd. (Gurgaon, Haryana, India), and Cathleen Shargay with Fluor Enterprises (Aliso Viejo, California, USA), research has shown that about 10 to 40% of corrosion costs can be avoided with appropriate engineering during the design phase and corrosion management during operation.
Many process streams in petroleum refineries with an aqueous phase contain enough hydrogen sulfide (H2S) to cause corrosion or cracking in susceptible materials. The most common types of cracking due to wet H2S are sulfide stress cracking (SSC), hydrogen-induced cracking (HIC), and stress-oriented hydrogen-induced cracking (SOHIC). Additionally, amine, caustic, and wet carbonate streams can cause stress corrosion cracking (SCC), depending on concentrations, temperatures, and other variables. Some services have a risk of wet
H2S
damage and SCC if multiple corrodents are present.
Desai, Murthy, and Shargay emphasize that adopting best engineering practices at the engineering design and construction stages is an essential step for controlling corrosion and corrosion-related cracking in a refinery. In a paper presented at CORROSION 2018,1 they describe several cracking-related damage mechanisms and discuss control measures to mitigate corrosion and cracking.
SSC is defined as metal cracking under the combined action of tensile stress and corrosion in the presence of water and H2S. SSC is a form of hydrogen stress cracking that results from the absorption of atomic hydrogen produced by the sulfide corrosion reaction on the metal surface. SSC in refining equipment is affected by the complex interaction of factors such as steel composition, material strength (as indicated by the hardness and microstructure of the material exposed to the sour environment), weld material and heat treatment, total tensile stress (applied and residual), hydrogen flux generated in the material due to surface corrosion, temperature (which primarily affects corrosion rates), and time. The primary step for avoiding SSC is to apply hardness limits to the base materials and welds.
HIC can occur in steel exposed to wet H2S conditions. This form of attack typically occurs in steel with relatively soft microstructures and commonly appears as blisters and/or stepwise cracking. HIC is caused by atomic hydrogen that diffuses into the steel and is trapped in internal defects such as laminations or nonmetallic inclusions (e.g., manganese(II) sulfide [MnS]), where it recombines to form molecular hydrogen (H2). Because H2 has a considerably greater volume than atomic hydrogen, it cannot diffuse through the steel, so pressure builds in the metal until cracking and deformation (blistering) of the steel occur. Important factors for avoiding HIC are purity and homogeneity of the steel (i.e., minimizing laminations and elongated nonmetallic inclusions).

SOHIC is characterized by a stack of small HIC blisters that are connected by cracks that travel through the thickness of the metal. The cracks are driven by a high level of either applied or residual stress that is perpendicular to the metal surface. Since residual stresses are highest in weld heat-affected zones (HAZ), one of the most important mitigation steps is postweld heat treatment (PWHT). Since SOHIC grows through the metal’s thickness, it reduces the load bearing capacity of the component.
Caustic SCC appears as intergranular cracking on carbon steel (CS) under the combined action of tensile stress and corrosion in the presence of sodium hydroxide (NaOH), potassium hydroxide (KOH), or other strong alkaline compositions at high temperatures. Amine SCC is a form of alkaline SCC that occurs under the combined action of tensile stress and corrosion in the presence of an aqueous alkanolamine liquid at various temperatures, and is predominately intergranular. Amine corrosion and SCC are typically a concern in amine treating and regeneration units, in which aqueous alkanolamine solutions are used to remove acid gases such as H2S and carbon dioxide (CO2) from various gas or liquid hydrocarbon streams. This type of cracking is most often found at or adjacent to CS weldments without a PWHT or in highly cold-worked components. Carbonate SCC is also a form of alkaline SCC that typically occurs close to or in the vicinity of welds; and some cracking has been reported up to 50 mm away from welds that have not received PWHT.
The fabrication practices recommended to avoid SSC, HIC, and SOHIC in wet H2S services can vary with the severity of the service. The severity can be categorized based primarily on characteristics of the liquid water present (continuous, intermittent, condensing, only from excursions, etc.); levels of corrosives such as H2S, hydrogen cyanide (HCN), H2, ammonium hydrosulfide (NH4HS), and CO2; the pH of the aqueous phase; and, if the component also handles amine, the amine type (monoethanolamine [MEA], diethanolamine [DEA], methyl diethanolamine [MDEA], etc.) and whether the amine is lean or rich. Table 1 can be used as a guide for identifying the category/ severity of service. These severity levels are similar to some of the options provided in NACE Publication 8X194,4 and there is a NACE committee currently working to achieve industry consensus on these categorizations.
Once the severity of wet H2S service is identified, various control measures can be set to mitigate corrosion risks associated with these corrosive services, as shown in Table 2. The first control measure is to establish requirements— chemistry restrictions, mill heat treatments, or additional requisites for the materials’ physical properties—for the raw materials selected for the various equipment components. For example, carbon equivalent (CE) has a direct relationship to the hardness of the HAZ. Base metal microalloying elements, such as vanadium, niobium, titanium, and boron, can also increase HAZ hardness and reduce the tempering effect of PWHT on the HAZ. Heat treatments for the base metal, such as normalizing (N), quenching (Q), or tempering (T), increase resistance to crack growth in severity conditions classified as moderate and high.
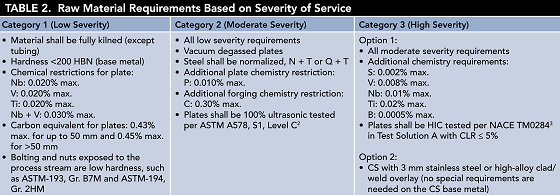
Susceptibility to hydrogen blistering and HIC in high severity services is primarily related to the quality of the plate steel (i.e., the number, size, and shape of the discontinuities). In this regard, the sulfur content of the steel is a key material property. Reducing the sulfur content of the steel selected for use in high severity service reduces its susceptibility to blistering and HIC. Table 2 lists the most commonly used CS material requirements used in wet H2S service based on the service’s severity level.
Another control measure involves fabrication guidelines such as heat treatment, testing, and inspection. Since hardness is the most important contributing factor for SSC mitigation and residual stress reduction is needed to prevent SCC and SOHIC, an effective PWHT (which reduces both hardness and residual stresses) should be determined. Most specifications and NACE SP04725 require equipment in wet H2S service to receive a PWHT at a temperature range of 620 °C minimum with a hold time of 1 h per 25 mm of thickness and a minimum of 1 h. Also, Desai, Murthy, and Shargay add that all cold-formed (>5% strain) components should be stress relieved even when not required by code.
To mitigate the risk of caustic SCC, all CS welds and cold-formed (≥5% strain) components in equipment or piping should receive stress relief/PWHT when the caustic concentration and maximum operating temperature exceed the curve shown in Figure 1 of NACE SP0403.6 The PWHT temperature should be 635 ± 14 °C for a minimum of 1 h, as recommended in NACE SP0403. As with caustic services, Desai, Murthy, and Shargay comment that all CS welds and cold-formed (≥5% strain) components in amine equipment or piping should also receive stress relief/ PWHT at a temperature of 635 ± 14 °C for a minimum of 1 h to mitigate the SCC risk.
Typical testing and nondestructive examination on completed components include weld deposit hardness testing and wet fluorescent magnetic particle testing. The production weld hardness is usually limited to a maximum of 200 Brinell hardness. For equipment, one hardness test is normally done every 3 m along the weld length on the main seams, with nozzle and manway welds tested on the process side (where accessible). For piping, a percentage of welds is generally tested, with the percentage based on the severity of the service.
For equipment prone to corrosion attack, Desai, Murthy, and Shargay also recommend developing process or operational controls that are typically applied as an integrity operating window (IOW), which is a set of limits that defines an assortment of variables (i.e., a velocity limit in amine services or temperature limit in caustic service) that can affect a process unit’s mechanical integrity and reliability. Damage or failure that is otherwise preventable can occur if a unit is operated outside of its IOW.
References
1 J.J. Desai, C. Shargay, A.S. Murthy, “Good Fabrication Stage Practices to Mitigate Risks Associated with Wet H2S, Caustic, Amine, Carbonate Corrosion and Cracking,” CORROSION 2018, paper no. 10842 (Houston, TX: NACE International, 2018).
2 ASTM A578/A578M–17, “Standard Specification for Straight-Beam Ultrasonic Examination of Rolled Steel Plates for Special Applications” (West Conshohocken, PA: ASTM, 2017).
3 NACE TM0284-2016, “Evaluation of Pipeline and Pressure Vessel Steels for Resistance to Hydrogen-Induced Cracking” (Houston, TX: NACE, 2016).
4 NACE Publication 8X194, “Materials and Fabrication Practices for New Pressure Vessels Used in Wet H2S Refinery Service” (Houston, TX: NACE, 2006).
5 NACE SP0472-2015, “Methods and Controls to Prevent In-Service Environmental Cracking of Carbon Steel Weldments in Corrosive Petroleum Refining Environments” (Houston, TX: NACE 2015).
6 NACE SP0403-2015, “Avoiding Caustic Stress Corrosion Cracking of Refinery Equipment and Piping” (Houston, TX: NACE, 2015).