
A new class of high-temperature alumina-forming austenitic (AFA) stainless steel (SS) alloys that deliver both superior corrosion and creep resistance for many industrial environments—without sacrificing the typical lower cost, formability, and weldability of conventional high-temperature SS—has been developed by researchers with the Oak Ridge National Laboratory (ORNL) (Oak Ridge, Tennessee). The outstanding corrosion resistance of these alloys is due to their unique composition, which allows the formation of a protective aluminum oxide (Al2O3 [alumina]) scale layer on the metal’s surface that provides significant oxidation resistance. This protective layer permits the use of these alloys at higher temperatures and for longer time periods than SS alloys that form a protective chromium oxide (Cr2O3 [chromia]) surface layer.
The particular corrosion-resistant attributes of these AFA SS alloys make them suitable for a wide range of energy production, chemical, and process industry applications, where the use of more durable materials capable of withstanding higher temperatures can result in significant cost and energy savings as well as reductions in environmental emissions. While some of these characteristics can be found in existing alloys, this new alloy can be produced at a lower price than other existing alloys that require large amounts of nickel.
According to NACE International member Michael P. Brady, distinguished R&D staff member with ORNL’s Materials Science and Technology Division, the AFA SS alloy family was developed as an alternative for chromia-forming SS and, under some circumstances, nickel (Ni)-based alloys in wrought plate, sheet, strip, foil forms, extruded tubes, and cast forms. In the high-temperature corrosive environments often found in energy conversion and combustion system applications as well as the chemical, petrochemical, and process industries, these alloys offer better corrosion resistance plus mechanical properties that are comparable to advanced chromia-forming austenitic alloys. “We see potential for AFA alloys in process environments involving aggressive water vapor, carbon [C], and sulfur [S] species in temperatures ranging from 500 to 900 °C,” he says. “Every application and environment is unique, and the alloys that perform well can vary significantly. For example, in water vapor and steam, AFA alloys do particularly well compared with chromia-forming alloys. In sulfidation-oxidation conditions encountered in petrochemical production, AFA alloys also show promise.”

Austenitic SS, the most common form of SS, has a face-centered cubic (FCC) crystal structure that is stabilized by nickel. It is easily formed and welded; exhibits better high-temperature creep strength than ferritic SS, which features a body-centered cubic [BCC] crystal structure; and can be used in a wide range of temperatures. Typically austenitic SS alloys contain between 16 to 25% chromium, which forms the Cr2O3 layer that supports their high resistance to corrosion. Heat-resistant austenitic SS and related iron-nickel (FeNi) alloys, because they combine good creep strength and oxidation resistance, are widely used in energy production and chemical processing environments in temperatures ranging from ~500 to 900 °C.
The aggressive water vapor, C, and S species encountered in many high-temperature process environments, however, can compromise the corrosion resistance of chromia-forming austenitic SS alloys because of rapid oxidation, Brady explains. At temperatures above ~600 °C, water vapor, a byproduct of combustion, results in the rapid formation of volatile Cr oxy-hydroxides that can significantly reduce the lifetime of chromia-forming alloys, particularly in thin section applications such as heat exchangers and turbine recuperators. Water vapor can also increase internal oxidation in chromia-forming alloys, which has a detrimental effect on the material’s durability.
Achieving targets for equipment endurance under these process conditions may require the use of Ni-base alloys, which can be more expensive than austenitic SS due to the high cost of nickel. Extending equipment service life may also necessitate the use of less aggressive process conditions (which can reduce process efficiency and increase environmental emissions) so that less expensive SS can be used. The development of the AFA SS alloy family was initially driven by the need for an austenitic SS with improved high-temperature corrosion resistance in the presence of water vapor and steam. The goal was to provide a solution for enhancing material durability without significantly increasing alloy cost.
Slow-Growing Alumina Scale
Brady comments that alumina scale grows at a much slower rate than chromia—one to two orders of magnitude slower—and is more thermodynamically stable in oxygen at elevated temperatures. Alumina scale is also more resistant to the effects of water vapor, and significant oxidation volatility isn’t typically observed until temperatures reach ~1,200 °C. This enables the alumina scale to provide a continuous, protective surface layer with superior high-temperature oxidation resistance. “You never stop corrosion, you just manage it,” Brady says. “So continued corrosion, which is the continued growth of the oxide layer, depends on how fast the oxygen penetrates the metal surface.”
 He notes that both alumina and chromia surface scale will form on SS within a couple of minutes in high-temperature environments. These protective layers resist oxygen penetration, which slows the corrosion reaction and the formation of iron and nickel oxides. From that point forward, Brady adds, the corrosion rate is determined by how fast the oxygen transports through the oxide layer to the metal surface and consumes the alloy’s components, which is the process that causes the oxide scale layer to grow. Alumina is expected to protect the surface much longer because it impedes oxygen transport and grows much more slowly than chromia in high-temperature process environments with aggressive oxidizing conditions, such as the presence of water vapor, C, and S species.
He notes that both alumina and chromia surface scale will form on SS within a couple of minutes in high-temperature environments. These protective layers resist oxygen penetration, which slows the corrosion reaction and the formation of iron and nickel oxides. From that point forward, Brady adds, the corrosion rate is determined by how fast the oxygen transports through the oxide layer to the metal surface and consumes the alloy’s components, which is the process that causes the oxide scale layer to grow. Alumina is expected to protect the surface much longer because it impedes oxygen transport and grows much more slowly than chromia in high-temperature process environments with aggressive oxidizing conditions, such as the presence of water vapor, C, and S species.
Generally, the addition of Al and Cr to steel to increase its corrosion resistance results in reduced creep strength. The more Al and Cr that is added, the more the steel’s creep strength is diminished. This happens because Al and Cr will stabilize the weak ferritic form of iron at the expense of the stronger austenitic form, which results in a loss of creep strength. Over the past 30 years, alloy developers worldwide have attempted to create alumina-forming, iron-based austenitic SS that is creep resistant. Ferritic Fe-Cr-Al alloys capable of forming a protective alumina scale layer have been available for specialty applications such as heating elements, furnace liners, and catalyst support beds, but these alloys are too weak for most high-temperature structural uses. Alumina-forming Ni-based alloys with excellent high-temperature creep resistance are also available, but are about three to five times more costly than austenitic SS due to the high cost of nickel.
Fine-Tuning the Alloy Composition
The researchers used computational thermodynamic calculations to assess potential alloy compositions with regards to crystal structure (FCC or BCC) and creep strength. Careful composition control to maintain the alloy’s austenitic crystal structure was an important aspect of the alloy’s development, Brady says, noting that there is a point where additions of aluminum and chromium to get corrosion resistance and the addition of nickel to gain creep strength must balance. The researchers also gained significant insights into alloy design by studying the oxidation of model and developmental alloys. They were able to identify a range of compositions, with relatively low Al and Cr contents (2.5 to 4 wt% and 14 to 15 wt%, respectively), that formed alumina scale and also permitted stabilization of an FCC austenitic matrix phase for creep strength with comparatively low additions of Ni (i.e., 12, 25, or 32 wt% depending on the AFA alloy grade).
They also discovered that adding minor amounts of niobium (Nb) helped promote alumina formation in these composition ranges. “That’s really why the alloy works,” Brady comments, explaining that additions of Nb, Ni, Cr, boron (B), and C all assist with alumina formation. “The behavior is quite complex and not yet fully understood.”
Three important experimental findings formed the foundation for the researchers’ successful development of the alloy, says Brady. First, protective alumina surfaces can be formed on austenitic SS with only 2.5 to 4 wt% Al and 12 to 15 wt% Cr (Cr aids in establishing the alumina surface). Although they are ferritic SS stabilizers, these relatively low levels of Al and Cr permit stabilization of a strong austenitic matrix microstructure with Ni levels that are comparable to conventional austenitic SS (~12 to 35 wt% Ni depending on Al/Cr content). Second, the addition of 0.6 to 3 wt% Nb synergistically enhances the formation of an alumina surface layer on Al-modified austenitic SS. Furthermore, the use of nitrogen (N), titanium (Ti), and vanadium (V), which are common strengthening additions, degrade the ability to form an alumina surface layer in the AFA composition range and must be minimized. Ti can be used under some circumstances, but must be countered by higher Ni levels. Third, good creep resistance can be achieved in AFA SS via nanocarbides and/or nanoprecipitates. AFA SS can also be strengthened by gamma prime precipitates (γ’-Ni3Al) with the proper balance of Al, Ni, Nb, and Ti additions.
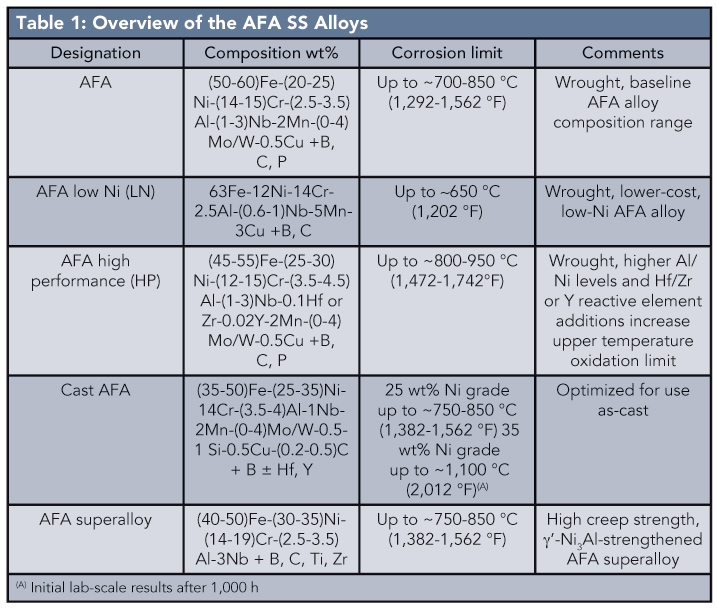
The researchers developed five distinct grades of AFA SS alloys (see Table 1) that provide an array of use-temperatures, creep strengths, and alloy cost depending on the grade, and can match the creep strengths of state-of the-art austenitic SS. “In effect, alumina-forming austenitic alloys bridge the gap in high-temperature corrosion resistance between austenitic stainless steels and nickel-based alloys, at relatively low alloy cost, while maintaining the good creep resistance and mechanical properties of austenitic stainless steels,” Brady says.
The alloys’ composition range includes Fe (35 to 63 wt%), Ni (12 to 35 wt%), Cr (12 to 15 wt%), Al (2.5 to 4 wt%), Nb (0.6 to 3 wt%), and manganese (Mn) (0.0 to 5 wt%). The Al, Cr, Mn, and Ni are balanced to maintain the austenitic microstructure, and carbide precipitates are incorporated for creep strength. “Once we understood how to make alumina-forming stainless steels, we looked to develop several different grades that could offer attractive properties at a range of costs,” Brady says.
For example, the AFA low Ni grade is based on 12 wt% Ni vs. the alloy’s baseline of 25 wt% Ni, and contains lower levels of Nb, molybdenum (Mo), and tungsten (W), which reduces the material costs by half those of the baseline AFA grade. While these composition changes for the low Ni grade cap its upper temperature limit to 650 to 700
°C for oxidation resistance, the lower cost makes it attractive for applications where less corrosion resistant austenitic SS grades are currently used.
Performance Studies
The AFA alloy family’s high-temperature corrosion and creep resistance have been extensively evaluated in laboratory studies.In general, the AFA alloys exhibited superior high-temperature corrosion resistance vs. chromia-formers in water vapor, steam, and sulfidation/oxidation conditions. Data based on mass change from oxidation in air with 10 vol% water vapor were collected for various AFA alloy grades that are comparable to commercially available, chromia-forming wrought and cast SS, and nickel-based alloys. The mass change data for the AFA alloys indicated slow, protective alumina scale formation with greater oxidation resistance than nickel-based chromia-forming alloys under these test conditions.

Creep data for AFA alloys relative to tabulated data for commercial chromia-forming wrought and cast alloys were also collected. The wrought AFA alloys showed creep strength properties comparable to advanced commercial austenitic SS, with some AFA alloy grade compositions approaching the creep strength of the alloy Grade TP310MoCbN (UNS S31025) in ASTM A213/A213M,2 which is generally considered to have one of the highest creep strengths available for an austenitic SS alloy. At 700 °C, initial data indicated the AFA superalloy grade exhibits creep strength approaching that for the nickel-based alloy UNS N06617. In laboratory trials, the AFA alloys in plate and sheet form were welded with little deficit in creep strength.
This is a significant finding, as widespread adoption of the AFA SS alloy family technology for industrial use requires that the alloys be weldable.
The AFA SS alloys are also being evaluated under a wide range of sulfidation, carburization, nitridation, and molten salt/deposit conditions. Overall, the AFA SS alloy family showed high-temperature corrosion resistance advantages for many, but not all, energy conversion, combustion system, and chemical/petrochemical process environments. Results indicate these alloys are not suitable under highly reducing and nitridation conditions due to rapid internal aluminum-nitride formation; mixed results were observed under carburization conditions, with performance depending on specifics of the environment; and poor results were seen for metal dusting resistance, with the exception of the AFA low Ni grade that showed promise in laboratory studies. In most molten salt/deposit conditions (e.g. hot corrosion), chromia-forming alloys with high Cr and Ni content fared better than the AFA SS alloys.
Currently, field tests of components fabricated with AFA SS are underway. Field exposures in progress range from simple coupons in chemical process environments to trial turbine recuperator components at an industrial site. AFA SS material also has been provided to universities for studying and publishing the findings on its microstructure, creep, and corrosion behavior. Brady notes that, upon request, ORNL will provide material samples for testing to others. “The more material we get out there to be evaluated, the better we will see where it works best and makes a positive impact,” he says.

Three wrought AFA alloy grades—the baseline grade, the low Ni grade, and the high-performance grade—were licensed to Carpenter Technology Corp. (Reading, Pennsylvania) in 2011. Trial 25- and 400-lb (11- and 181-kg) heats, as well as a 10,000-lb (4,536-kg) production heat run by Carpenter Technology indicate these wrought AFA alloy grades can be readily hot and cold rolled into a variety of plate, sheet, strip, and foil product forms by standard metallurgical processing.The wrought grades are currently available as precommercial products for industrial trials and field tests.
Recent developments include a cast AFA SS alloy composition with a 35 wt% Ni base that shows promise for operating in temperatures in the 1,100 °C range, Brady says. “We hope to pursue development of this AFA for ethylene cracking environments, where alumina scale may offer greater resistance to coking effects,” he adds. ORNL and Carpenter Technology are also considering the pursuit of ASME boiler and pressure vessel code case approval for using the AFA SS alloys in high-pressure environments.
Developers of the AFA SS alloys are Michael P. Brady, Yukinori Yamamoto, Bruce A. Pint, Govindarajan Muralidharan, Philip J. Maziasz, Michael Santella, Zhaoping Lu, and Chain-tsuan Liu. This technology, a collaboration between ORNL and John H. Magee, Samuel J. Kernion, David A. Helmick, and Timothy R. Armstrong with Carpenter Technology Corp., is the recipient of a 2015 MP Corrosion Innovation of the Year Award as well as a 2009 R&D 100 Award. More information about the AFA SS alloys can be found at the ORNL Web site, ornl.gov.
References
- M.P. Brady, G. Muralidharan, D. N. Leonard, J.A. Haynes, R.G. Weldon, R.D. England, “ Long-Term Oxidation of Candidate Cast Iron and Stainless Steel Exhaust System Alloys from 650-800°C in Air with Water Vapor,” Oxidation of Metals 82, 5-6 (2014): pp. 359-381.
- ASTM A213/A213M-15a, “Standard Specification for Seamless Ferritic and Austenitic Alloy-Steel Boiler, Superheater, and Heat-Exchanger Tubes” (West Conshohocken, PA: ASTM International, 2015).
Bibliography
Brady, M.P., J. Magee, Y. Yamamoto, D. Helmick, and L. Wang. “Co-Optimization of Wrought Alumina-Forming Austenitic Stainless Steel Composition Ranges for High-Temperature Creep and Oxidation/Corrosion Resistance.” Mater. Sci. Eng. A: A 590 (2014): pp. 101-115.
McGuire, M.F. Stainless Steels for Design Engineers. Materials Park, OH: ASM International, 2008.
Muralidharan, G., Y. Yamamoto, M.P. Brady, B.A. Pint, D. Voke, and R.I. Pankiw. “Development of Cast Alumina-forming Austenitic Stainless Steel Alloys for use in High Temperature Process Environments.” CORROSION 2015, paper no. 6114. Houston, TX: NACE International, 2015.
Roberto, J.B. “Alumina-Forming Austenitic Alloy Family.” MP Corrosion Innovation of the Year Awards’ 2015 Award Nomination, Oakridge National Laboratory. October 2014.
Unocic, K.A., M.P. Brady, and B.A. Pint, “Oxidation Behavior of Alumina-Forming Austenitic Steel.” CORROSION 2013, paper no. 2172. Houston, TX: NACE International, 2013.
