Contamination of organic chloride compounds in crude has become a source of deep concern for oil consumers and refinery operators. Organochloride compounds are organic substances containing at least one covalently bonded atom of chloride (R-Cl). These include, but are not limited to, chloroform (CHCl3) and dichloromethane (CH2Cl2). These compounds are effective solvents for cleaning and removing paraffin, deposits, and other degreasing operations.
The crude oil can be contaminated by the organochloride compounds from different processes, whether upstream processes and/or within the refinery processes. They can be hydrolyzed in a crude distillation unit during the heating process to form hydrochloric acid (HCl) resulting in extensive and dangerous corrosion. This contamination leads up to severe damage in refinery process equipment, which results in shutdown of some units or the entire refinery. It is well documented in the literature1-8 that the contamination of organic chloride compounds in crude is the root cause of hydrochloric acid attack that impacts the corrosion experience in naphtha hydrotreater unit (NHT) effluent systems due to excess organic chloride in the NHT feed.
Our earlier research work1 discussed the experience with low pH in spent wash water from a separator water/product boot in the NHT that contributed significantly to severe corrosion in carbon steel (CS) tubes of the air-cooled condenser. Figure 1 shows a diagram of the NHT process illustrating that corrosion incident. The root cause of this severe corrosion mechanism was attributed to the presence of a high organochloride concentration in the naphtha feed to the NHT unit.
The authors have not identified data in the technical literature or other laboratory works that confirm an acceptable concentration of organic chloride in the naphtha feed that would not drop pH in sour water that causes severe corrosion of the product separator. This article describes the effect of organic chloride concentrations in naphtha on corrosion behavior of CS by simulating the NHT unit conditions.
Organic Chloride Partitioning in Refined Products
The reported chloride in crude oil is related to the total chloride, which includes both inorganic chloride and organic chloride. In the case of organic chloride contamination of a crude, there is no direct method to measure organic chloride concentration in that material. The crude needs to be distilled first into several cuts and each cut should be washed with deionized water several times before the analysis. Then, measured chloride using the UOP991 standard9 is assumed to be organic chloride, where all inorganic chloride salts were removed by the desalting water wash procedure before the analysis.
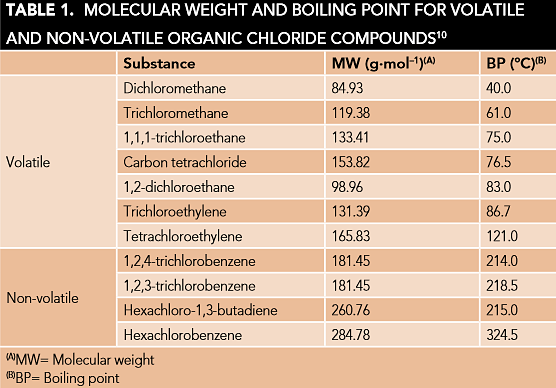
However, most of the reported organic chloride in a crude oil was based on testing of only the naphtha cut. It is worth mentioning that there are some nonvolatile organic chloride compounds with high boiling points (Table 1)10 that can be partitioned into heavier hydrocarbon streams if they are present in the crude and it should also be considered in the chloride analysis. There is a scarcity of articles11-12 on characterization of high boiling organic chlorides with complex molecular structure in heavy fractions of petroleum, such as atmospheric residual (AR) and vacuum gas oil.
Recent work13 shows analysis of organic chlorinated compounds in the AR of crude oil, including three organic chlorinated compounds with a chlorobenzene core structure. DL-p-chlorophenylalaninol, phthalic acid, 2-chlorophenyl ethyl ester, and 2H-1,2-oxazine, 6-(4-chlorophenyI) tetrahydro-2-methyl- were identified in the AR. This work focuses on partitioning a volatile organic chloride compound in crude and its corrosion impact on CS material.
Experimental Procedure
To understand concentration distribution of organic chlorinated compounds in different distillates, an examination was made for two common organic chlorides used in refineries and upstream processes, namely tetrachloroethylene (C2Cl4) (PCE, boiling point = 121.1 °C) and trichloroethylene (C2HCl3) (TCE, boiling point = 87.2 °C). Artificial organic chloride contamination of crude oil was conducted in a laboratory where 10 ppm of each chemical was injected in 300 mL of a crude that did not contain any organic chlorides.
Trichloroethylene and tetrachloroethylene are used extensively in upstream and downstream for chemical cleaning process and catalyst reactivation processes due to their cost and effectiveness. Because of this, they were selected for this study.
Then, the contaminated crude was distilled into several side cuts in accordance with ASTM D1160.14 The concentration of organochlorides in each distillate was determined using a gas chromatograph connected to an electron capture detector. The system was fitted with a nonpolar chromatographic column (DB1†, 60 m long, 0.32 mm ID, and 1.0 µm film thickness). The column was operated under a constant helium carrier gas with a flow of 1.0 mL/min. The gas chromatography oven was ramped from 40 to 310 °C at a rate of 10 °C/min. The quantification was achieved by external calibration while establishing for each compound the relation between the concentration and the peak areas. For both compounds, a good linear correlation was obtained in the 0.3 to 200 ppm range (R2 > 0.999).
The effect of organic chloride concentration in the naphtha feed on the corrosion behavior of CS was studied by simulating the conditions of an air-cooled condenser in the NHT using a high-pressure/high-temperature (HPHT) autoclave reactor. The corrosion mechanism in the NHT is HCl attack, as is well documented in the literature. Therefore, as a first step the generated HCl, which is a result of reaction between chloride dissolution from organic chloride compounds and hydrogen, in the NHT was simulated. The ratio of naphtha to water was 9:1. The experiments were conducted at 300 °C and 800 psi, similar to the NHT reactor conditions, for 4 h, including cooling and heating time, and the tested concentration of each chemical was between 0.5 to 1,200 ppm.
After test termination, the water was collected to measure pH and chloride concentration in accordance to ASTM D1293-1215 and ASTM D4327-11.16 The HPHT autoclave corrosion tests (ASTM G17017) were carried out immediately on coupons made of CS 1018 in the collected water at the inlet conditions of air-cooled condenser, which are 110 °C and 150 psi. The flow rate and the exposure time were 1 m/s and 5 h. The corrosion rate was measured using the weight loss method where initial and final weight of the exposed coupons were recorded.
Partitioning of TCE and PCE in Distillates
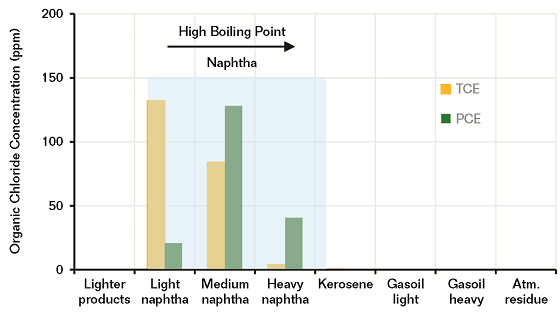
Figure 2 shows the partitioning results of each distillate. The results reveal that the 10 ppm of each chemical injected in the crude was concentrated in the naphtha cuts with respect to its boiling point. As expected, due to the low boiling point (87 °C), the TCE partitioned more in light naphtha, 132 ppm; 84 ppm in medium; and 4 ppm in heavy naphtha. The PCE partitioned more in medium, 128 ppm; 21 ppm in light; and 41 ppm in heavy naphtha. Less than 1 ppm of each chemical partitioned in kerosene cut.
Influence of Organic Chloride on Corrosion Behavior of Carbon Steel
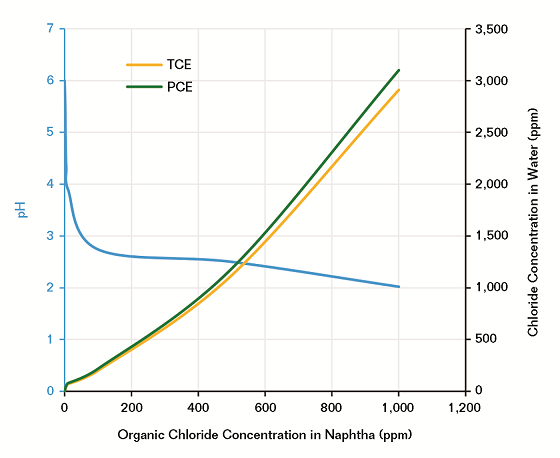
After generating the HCl through simulating the NHT reactor conditions in a HPHT autoclave, the pH and chloride content were measured in the extracted water against organic chloride concentrations, as shown in Figure 3. It was found that 10 ppm of an organic chloride compound in naphtha is enough to drop the pH to four and the chloride concentration in water increases proportionally with increasing organic chloride in the naphtha. For example, 500 ppm of organic chloride compound in naphtha produces 1,100 to 1,200 ppm chloride in water for TCE and PCE, respectively, a pattern that trends with respect to the number of chlorides in their chemical structure (C2HCl3 and C2Cl4).
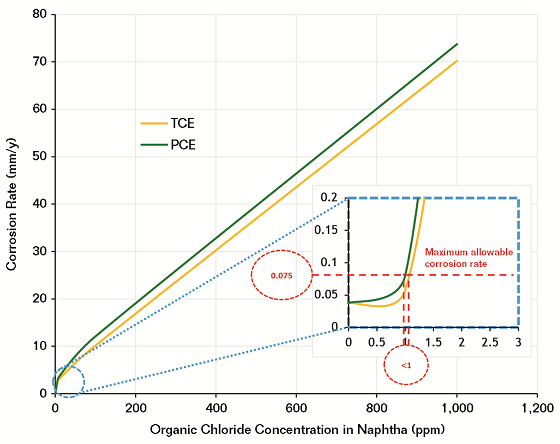
The corrosion behavior of CS was studied in those extracted waters to measure corrosion rate at different concentrations of each organic chloride compound separately in naphtha (Figure 4). It is obvious the corrosion rate increases tremendously with increasing the concentration of organic chloride compound in naphtha. If we assume that a crude oil contaminated with 10 ppm of TCE, then the concentration of TCE in naphtha will be 220 ppm, which can generate a high corrosion rate ~17 mm/y (670 mpy), which no system can tolerate.
Corrosion in the NHT system is not routinely monitored during normal operation. However, some literature reviews3,8 and experience in refinery corrosion with overhead systems reveal the acceptable corrosion rate is ~0.075 mm/y (3 mpy). It indicates that the organic chloride compound in naphtha should not exceed 1 ppm in order to control corrosion in a NHT system; otherwise, the system will be exposed to very corrosive water.
Conclusions
This work investigates the partitioning effect of two volatile organic chloride compounds commonly used as solvent at upstream and downstream operation processes on the corrosion behavior of CS.
The results indicate both chemicals concentrate almost totally in the naphtha cuts where the partitioning concentration is almost 19 to 22 times that injected in the crude, according to the number of chlorides in the different chemical structures. Acidity and chloride content in spent water increase severely with increasing organic chloride concentration in the naphtha. The corrosion mechanism was simulated by a HPHT autoclave using NHT operating conditions. The results confirm that the concentration of organic chloride in the naphtha should not exceed 1 ppm for corrosion control and maintaining the NHT integrity.
† Trade name.
References and About The Authors