Deposition of unwanted materials, including mineral scales, suspended matter, microbiological growth, and corrosion products, continues to plague the operation of industrial water systems. This article presents performance data on polyamino polyether methylene phosphonic acid (PAPEMP) on various mineral scales commonly encountered in boiler, cooling, desalination, geothermal, gas, and oil systems.
Water that is available for domestic and industrial applications typically contains many impurities. These impurities are generally classified in five broad categories:
• Dissolved inorganic compounds (i.e., carbonates, sulfates, phosphates, and fluorides of calcium, magnesium, barium, and strontium; small amounts of copper [Cu], iron [Fe], and manganese [Mn]); and other substances
• Dissolved gases (e.g., oxygen [O2], nitrogen [N2], carbon dioxide [CO2], and hydrogen sulfide [H2S])
• Suspended matter (e.g., clay, silt, fat, and oil)
• Soluble organic compounds (e.g., humic acid, fulvic acid, and tannic acid)
• Microorganisms (e.g., algae, bacteria, and fungi)
The accumulation of unwanted deposits on equipment surfaces is a phenomenon that occurs in virtually all processes in which untreated water is heated. The deposition of these materials, especially on heat exchanger surfaces in boiler, cooling, geothermal, and distillation systems, can cause a number of operational problems such as plugged pipes and pumps, inefficient use of water treatment chemicals, increased operational costs, lost production due to system downtime, and ultimately heat exchanger failure.1 Greater water conservation has been a driver for operating industrial water systems at higher concentration cycles, which increases the potential for deposit buildup on heat exchanger surfaces. Operating industrial water systems under stressed conditions demands a better understanding of the feed and recirculating systems’ water chemistry as well as the development of innovative additives and technological approaches for controlling scale, deposit, corrosion, and biofouling.
The most promising scale control method among various approaches involves adding substoichiometric dosages, typically a few ppm, of water-soluble additives to the feedwater. Additives commonly used in water treatment formulation fall into two categories:
• Dissolved inorganic compounds (i.e., carbonates, sulfates, phosphates, and fluorides of calcium, magnesium, barium, and strontium; small amounts of copper [Cu], iron [Fe], and manganese [Mn] ions; and other substances)
• Polymeric (e.g., homopolymers of acrylic acid, maleic acid, itaconic acid, aspartic acid, and copolymers containing monomers of different functional groups)
Although there are many phosphonates available, three of the most commonly used phosphonates in water treatment formulations are aminotrismethylene phosphonic acid (AMP); 1-hydroxyethylidine, 1,-1 diphosphonic acid (HEDP); and 2-phosphono-butane 1,2,4-tricarboxylic acid (PBTC). However, under certain pH, concentration, and temperature conditions, phosphonates have been shown to precipitate in the presence of calcium ions. The precipitation of calcium phosphonate salts not only creates fouling of heat exchanger and reverse osmosis (RO) membrane surfaces, it also decreases the solution concentration of a phosphonate to such an extent that severe calcium carbonate (CaCO3) scaling can occur.1-2 The focus of this study is to evaluate the performance of polyamino polyether methylene phosphonic acid (PAPEMP) as an inhibitor for various scales (e.g., CaCO3, calcium sulfate dihydrate [CaSO4•2H2O], and calcium phosphate [Ca3(PO4)2]) and a stabilization agent for Fe(III) or Fe3+ ions.
Experimental Protocols
All chemicals were obtained from commercial sources. They include AMP, HEDP, PBTC, 2-hydroxyphosphono acetic acid (HPA), PAPEMP, and polyacrylic acid (PAA). Detailed procedures for reagents solution preparation; percent inhibition (%I) calculation for calcium sulfate dihydrate (CaSO4•2H2O), CaCO3, Ca3(PO4)2, and Fe3+ stabilization; and instruments used are reported elsewhere.3-6 Table 1 lists the inhibitors tested.
Prevention of Mineral Scales
Calcium Sulfate Dihydrate
Considerable attention has been given to the various forms of CaSO4 crystallizing from aqueous solutions as affected by temperature, pH, solution stoichiometric ratio of lattice ions, and impurity level. These and other important factors involved in the nucleation and growth of CaSO4 •2H2O (gypsum), hemihydrate (CaSO4•0.5H2O) (plaster of Paris), and anhydrite (CaSO4) should have direct application to the control and inhibition of scale formation in industrial water systems. To preclude excessive costs, scale formation has to be prevented.
The effect of low concentrations (few ppm) of polymeric inhibitors on both the growth rate and crystal modification of gypsum has been investigated by several researchers. Amjad3 showed that polymers containing carboxyl groups (i.e., polyacrylic acid, polymaleic acid, polyitaconic acid) are particularly effective as gypsum growth inhibitors. Dogan, et al.7 arrived at similar conclusions after studying the effect of various acrylic acid-based copolymers as gypsum scale inhibitors.
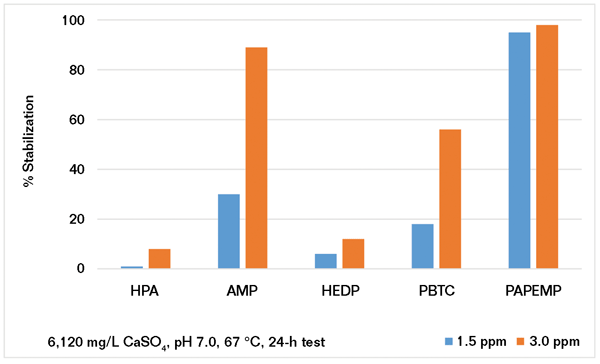
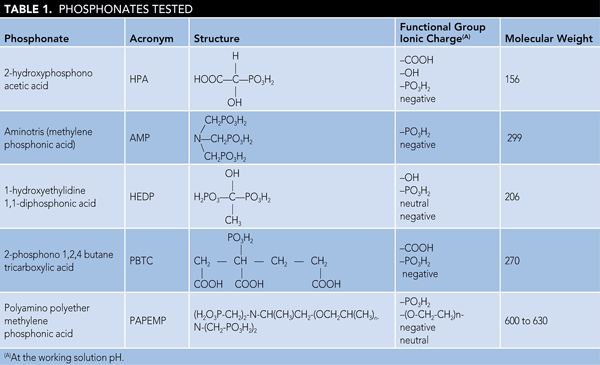
Figure 1 presents %I data for various phosphonates at 1.5 and 3.0 mg/L dosages, respectively. There are two points worth noting: a) the gypsum inhibition value increases with increasing inhibitor dosage and b) the inhibition value depends upon the inhibitor architecture. Phosphonates (i.e., HPA, HEDP) containing one (-OH) and one (-PO3H2) group, as shown in Table 1, exhibit poor performance as gypsum inhibitors when compared to AMP (containing three [-PO3H2] groups). For example, %I values obtained for HPA and HEDP at 3.0 ppm dosage are 8 and 12%, respectively, compared to 89% obtained for AMP.
Results on the performance of PBTC and PAPEMP are also presented in Figure 1. When compared to PBTC, PAPEMP exhibits excellent performance as a gypsum inhibitor. For example, %I values obtained in the presence of 1.5 ppm PBTC are 18% compared to 95% obtained for PAPEMP. Based on the data presented in Figure 1, phosphonate performance can be ranked as follows: PAPEMP > AMP > PBTC > HEDP > HPA.
Calcium Carbonate
CaCO3 is very often found in scale deposits, both in colloidal and amorphous states and in the form of polymorphs. The presence of relatively high concentrations of calcium and carbonate ions in water, in combination with the inverse solubility of CaCO3, is the main cause for the formation of this deposit. A particular and often complicating feature in the CaCO3 system is the polymorphism. Some of the various CaCO3 polymorphs encountered in aqueous media, in order of decreasing solubility, are calcium carbonate monohydrate, vaterite, aragonite, and calcite. Calcite is the most stable phase thermodynamically, although it is frequently encountered and identified as the sole component of CaCO3 scale formations and may result from the formation of less stable vaterite.
Commonly used inhibitors to control CaCO3 scale formation may be classified in the following broad categories: polyphosphates, polyphosphonates, synthetic polymers, natural polymers, and proprietary formulations. Polyphosphates have been known as efficient threshold inhibitors in the sense that they can inhibit CaCO3 scale formation at substoichiometric levels. They have been widely used despite their main disadvantage, which is hydrolysis of the esoteric P-O bond that yields orthophosphate and, upon further reaction with calcium ions, results in the formation of tenacious Ca3(PO4)2 deposits. Organophosphonates have been shown to be excellent CaCO3 inhibitors. These compounds, however, lead to the formation of calcium phosphonate salts under high hardness conditions. It is interesting to note that PAPEMP, compared to AMP, HEDP, PBTC, and HPA, has been reported to be more tolerant to Ca ions.8
As previously discussed, many systems are operating under stressed conditions (i.e., alkaline pH, high temperature, and high hardness levels) due to water shortages. To study the performance of phosphonates under stressed conditions, several experiments were carried out. The precipitation of CaCO3 was investigated under two different scaling conditions (SI-1 and SI-2). Data presented in Figure 2 show that the performance of phosphonates strongly depends on solution supersaturation. At low CaCO3 supersaturation (i.e., saturation index [SI] = 1.67),4 AMP and HEDP outperform PBTC, HPA, and PAPEMP. However, the performance of these phosphonates is quite different under stressed conditions (SI = 2.2). Results presented in Figure 2 show that all phosphonates provide good to mediocre performance at 10 ppm as CaCO3 inhibitors. As illustrated, increasing the dosage from 10 to 40 ppm shows a slight decrease in the performance of all phosphonates except for PAPEMP. The observed behavior of phosphonates may be explained by calcium phosphonate salt formation, which effectively decreases the solution concentration of phosphonates. Based on the data presented for the high SI system, the phosphonate ranking is: PAPEMP > PBTC ≈ AMP
≈
HPA
≈
HEDP. It is interesting to note that a similar trend has been reported for calcium ion tolerance of phosphonates.9
Calcium Phosphate
During the last two decades, the problem of Ca3(PO4)2 scaling has become increasingly important. Higher orthophosphate levels are being encountered in cooling water due to the use of low-quality makeup water, increased water reuse, and the use of organophosphonate as a corrosion and scale inhibitor, which degrades to orthophosphate. The increased orthophosphate levels, combined with alkaline operating conditions, can lead to the formation of insoluble Ca3(PO4)2 scale that is normally attributed to hydroxyapatite (HAP). In cooling water systems, however, it is not the HAP that is initially formed; instead a precursor phase is formed, which is widely known as amorphous Ca3(PO4)2 (Ca/P).
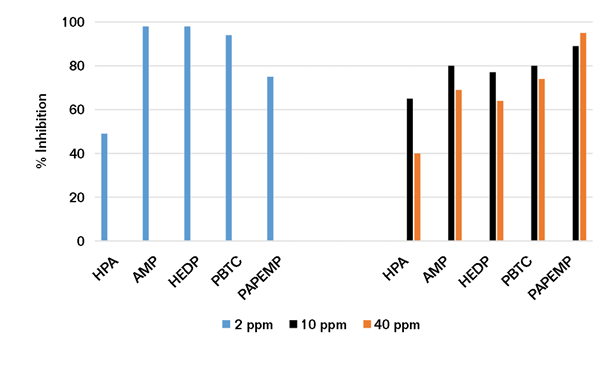
Using the experimental protocol described earlier, a series of Ca/P precipitation experiments was performed in the presence of 10 and 50 ppm of phosphonates. Data presented in Figure 3 reveal that phosphonates exhibit poor to mediocre (<40%) performance at 10 ppm concentrations. Increasing the phosphonate concentration fivefold—from 10 to 50 ppm—results in a significant decrease (~30%) in HPA, AMP, HEDP, and PBTC performance. Under similar conditions, PAPEMP performance is significantly improved—from 42 to 93%.
Based on the data presented, the effectiveness of phosphonates as Ca3(PO4)2 inhibitors follows the trend PAPEMP >> HPA, AMP, HEDP, and PBTC. As discussed before, the observed trend in phosphonate performance may be attributed to differences in calcium ion tolerance of these phosphonates.
Iron Stabilization
Among the various dissolved impurities in natural waters, metal ions, when present at a few ppm, pose the most serious problems in many domestic and industrial applications. These metal ions, including aluminum, copper, iron, manganese, and zinc, form insoluble hydroxides under acidic and/or alkaline conditions and deposit on equipment surfaces. They also influence the performance of scale inhibitors and dispersants commonly used in water treatment formulations. Removal of these heavy metal ions from industrial wastewater is of primary concern because they cause contamination of water bodies and are also toxic to many life forms.
In aqueous solutions, depending upon the water chemistry, ferric ions (Fe3+) form a variety of both soluble and insoluble complexes. The iron stabilization used in this experiment refers to the ability of the phosphonates to form soluble complexes, inhibit the formation of hydroxocomplexes, and/or disperse. In practice, it may be difficult to differentiate between truly soluble and very finely dispersed particles. Under the conditions employed, % stabilization is defined as the concentration of the ionic species that is not removed by filtration through a 0.22-µm filter.
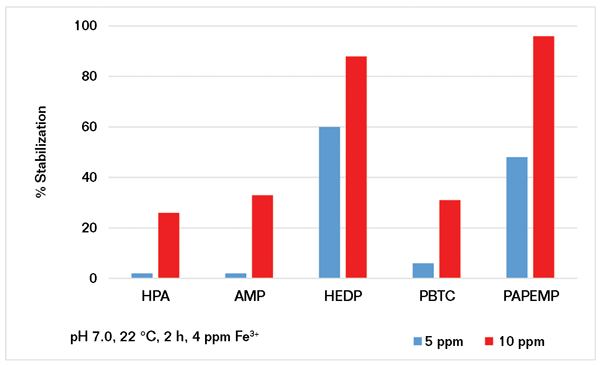
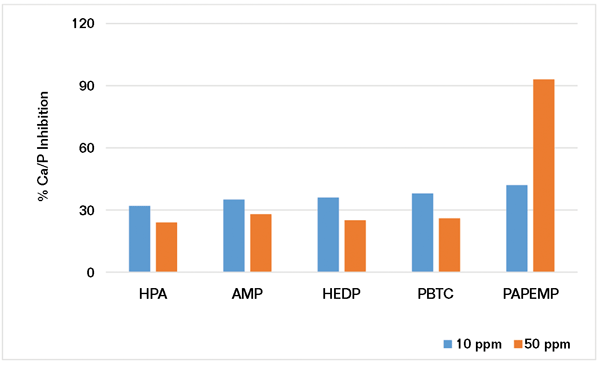
Using the experimental protocol described previously, several iron stabilization experiments were carried out in the presence of 5 and 10 ppm of phosphonates. Results presented in Figure 4 clearly show that iron stabilization strongly depends upon phosphonate concentration and architecture. Based on the data presented, the phosphonates can be ranked as follows:
• At 5 ppm: HEDP>PAPEMP>>PBTC, AMP, and HPA
• At 10 ppm: PAPEMP>HEDP>PBTC, AMP, and HPA
Conclusion
Based on the results of this study, PAPEMP, in optimum concentrations, provided superior control of calcium-based scale deposition and superior iron stabilization in natural waters when compared to the other phosphonates tested.
References
1 Z. Amjad, K. Demadis, eds., Mineral Scales and Deposits: Scientific and Technological Approaches (Amsterdam, The Netherland: Elsevier, 2015).
2 A.T. Kan, et al., “A Mechanistic Interpretation of the Precipitation and Dissolution of Divalent Metal Phosphonates,” CORROSION/93, paper no. 459 (Houston, TX: NACE International, 1993).
3 Z. Amjad, “Gypsum Scale Inhibition Using Biopolymers and Synthetic Polymers,” MP 51, 10 (2012): pp. 48-52.
4 Z. Amjad, J. Penn, “Impact of Iron Oxide (Rust) on the Performance of Calcium Carbonate Inhibitors, Part 1,” MP 53, 11 (2014): pp. 52-55.
5 Z. Amjad, “Calcium Phosphate Scale Control in Industrial water Systems,” Water Treatment J. 1-2 (2010): pp. 7-15.
6 Z. Amjad, “Influence of Polymer Architecture on the Stabilization of Iron and Manganese Ions in Aquesous Systems,” Tens. Surf. Det. 44, 4 (2007): pp. 202-208.
7 O. Dogan, et al., “Polyelectrolytes Inhibition Effect on Crystallization of Gypsum,” Crys. Res. Technol. 39 (2004): pp. 1,108-1,114.
8 J. Gill, “A Novel Inhibitor for Scale Control in Water Desalination,” Desalination 124 (1999): pp. 43-56.
9 Z. Amjad, et al., “Calcium Sulfate Dihydrate (Gypsum) Scale Inhibition by PAA, PAPEMP, and PAA/PAPEMP Blend,” Int. J. Corros. Scale Inhib. 3, 1 (2014): pp. 35-47.